I14 Control room:
Tel: +44 (0) 1235 778570
Principal Beamline Scientist:
Julia Parker
E-mail: [email protected]
Email: [email protected]
Tel: +44 (0)1235 778523
With the continued escalating of industrial application and production of engineered nanomaterials (ENMs), there arises the responsibility for understanding, mitigating, and reducing the ecological risks of these nanoscale entities. Specifically, their incorporation into commercial goods, followed by their disposal, may have unexpected environmental consequences. While there are concerns regarding the environmental ramifications of the increase of ENMs production, real evidence remains deficient for robust risk assessments. Predominantly, research projects have studied pristine (raw) materials, with only a small number of works studying the fate, persistence, translocation across mediums, and the temporal kinetics of ENMs. It has been stablished that the physicochemical characteristics of ENMs can undergo considerable modifications, contingent upon the encountered environments (e.g., via processes such as agglomeration, aggregation, speciation, and varied chemical transformations). In ecological contexts, ENMs undergo dissolution, modulate their surface chemistries (e.g., through ligand binding), aggregate, undergo chemical transformations, and release ions.
Consequently, it is pivotal to accurately characterise the materials before use, in different environments and at the nanomaterial-environment interface, to evaluate their environmental impact. In addition, there is an urgent need for studies which combine meticulous particle characterisation and sophisticated imaging techniques under realistic experimental scenarios. Paired with bioactivity assays into organisms, this information could provide a greater insight into ENMs behaviour and a better appreciation of potential effects on the environment.
With a beam size of about ~50 nm, the hard X-ray nanoprobe (I14 beamline) at Diamond offers the capability to monitor dynamic changes to the morphology and surface chemistry of the ENMs in situ within relevant hydrated environments, and with an outstanding combination of energy and spatial resolution, by X-ray fluorescence (XRF) microscopy. In addition, the chemical speciation of the transformed species can be interrogated by X-ray spectroscopy near-edge structure (XANES) analysis afterwards (Fig. 1).
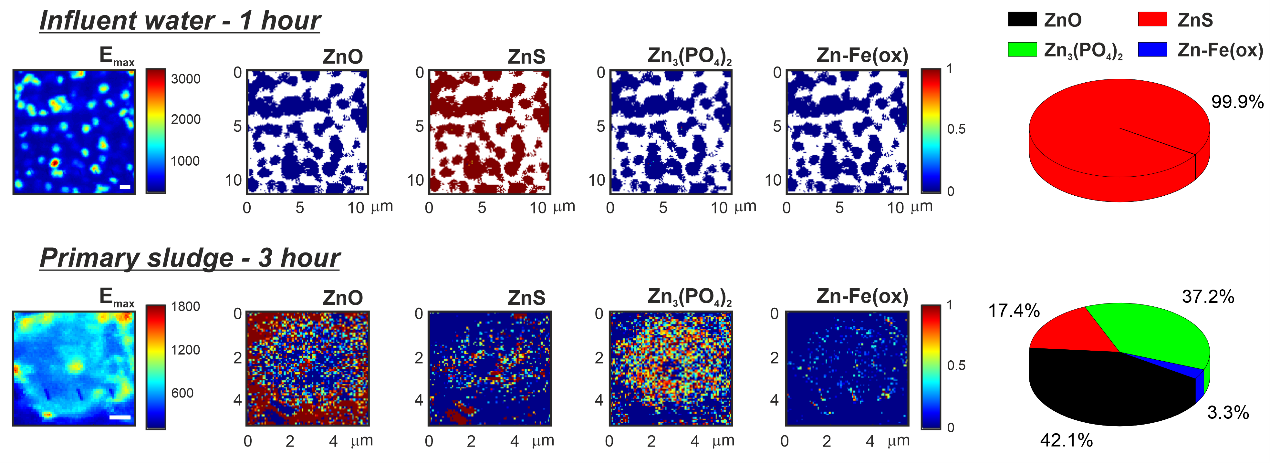
Figure 1.- XRF microscopy data of ZnO nanorods incubated in influent water for 1 hour (top) and in primary sludge for 3 hours (bottom). [Left] Fluorescence image acquired at the maximum of Zn K-edge (Emax = 9669 eV). [Middle] Speciation maps were calculated by XANES for the expected Zn- species: ZnO, ZnS, Zn3(PO4)2, and Zn adsorbed to Fe-oxyhydroxides (Zn-Fe(ox)) (from left to right), where the red colour equals a 100% contribution, and the blue colour corresponds to 0% (being the white pixels pure background – no-Zn detected). [Right] Pie charts with the average of all individual (pixel-by-pixel) percentages were also generated, representing the main contribution of each Zn-species. All the scale bars (white and black) are equal to 1 mm. © 2021 The Authors. Advanced Sustainable Systems published by Wiley-VCH GmbH. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited: Gomez-Gonzalez et al. Adv. Sustainable Syst. 2021, 2100023 (DOI: 10.1002/adsu.202100023).
Plastic litter has become a pressing environmental issue because the rate of disposable plastic manufacture has overcome our ability to deal with and recycle it efficiently. This has unknown consequences such as their breaking down into hard-to-detect micro- or nanoplastics (MPs/NPs). The resulting uncontrolled introduction of these emerging pollutants into the environment can have a significant effect on ecosystems, even though they may only be present at low concentrations.
We have already demonstrated that both pristine ZnO nanomaterials and ZnO naturally leached/released from a commercial sunscreen, effectively adsorbs into the surface of polystyrene microplastics (spherical, ~900 mm diameter) under mild incubations in freshwater systems. This leaves open the question of whether these MPs may act as transportation vector for co-existing nanomaterials (Fig. 2). In addition, a facial exfoliating scrub was also leached under the same conditions and incubated with pristine ZnO nanomaterials, also revealing the Zn-adsorption into the microplastics arisen from the exfoliating cleanser.
Developing powerful analytical instrumentation, which can detect trace concentrations of these MPs and their surface chemistries is vital to measure their lifetime and partitioning within the environment. Understanding their lifetime, and potential chemical modifications (and their kinetics), and evidencing which types of transformed nanomaterials are most environmentally harmful, would inform policy and regulations for their manufacture and use both nationally and internationally. Transforming Zn-species were characterised by XANES spectroscopy at the nanoscale in the published paper Gomez-Gonzalez et al. Global Challenges. 2023, 2300036), overall showing mixtures of incipient Zn-sulfide particles initially formed (kinetically favoured) and Zn-phosphate in the more accessible sites (thermodynamically preferred).
The I14 beamline is committed to unveiling the kinetics and dynamics of micro- and nanopollutants within in situ experiments under realistic environmental conditions, using to this end cutting-edge microscopic and spectroscopic techniques. It is precisely the complexity of studying MPs interaction with co-contaminants at the point of exposure, which makes difficult to extrapolate conclusions widely applicable. Furthermore, the vast diversity of MPs and nanomaterials currently disposed of means that a case-by-case analysis is impractical; therefore, there is an urgent need to develop guiding principles for predicting the risk of these pollutants in different contexts.
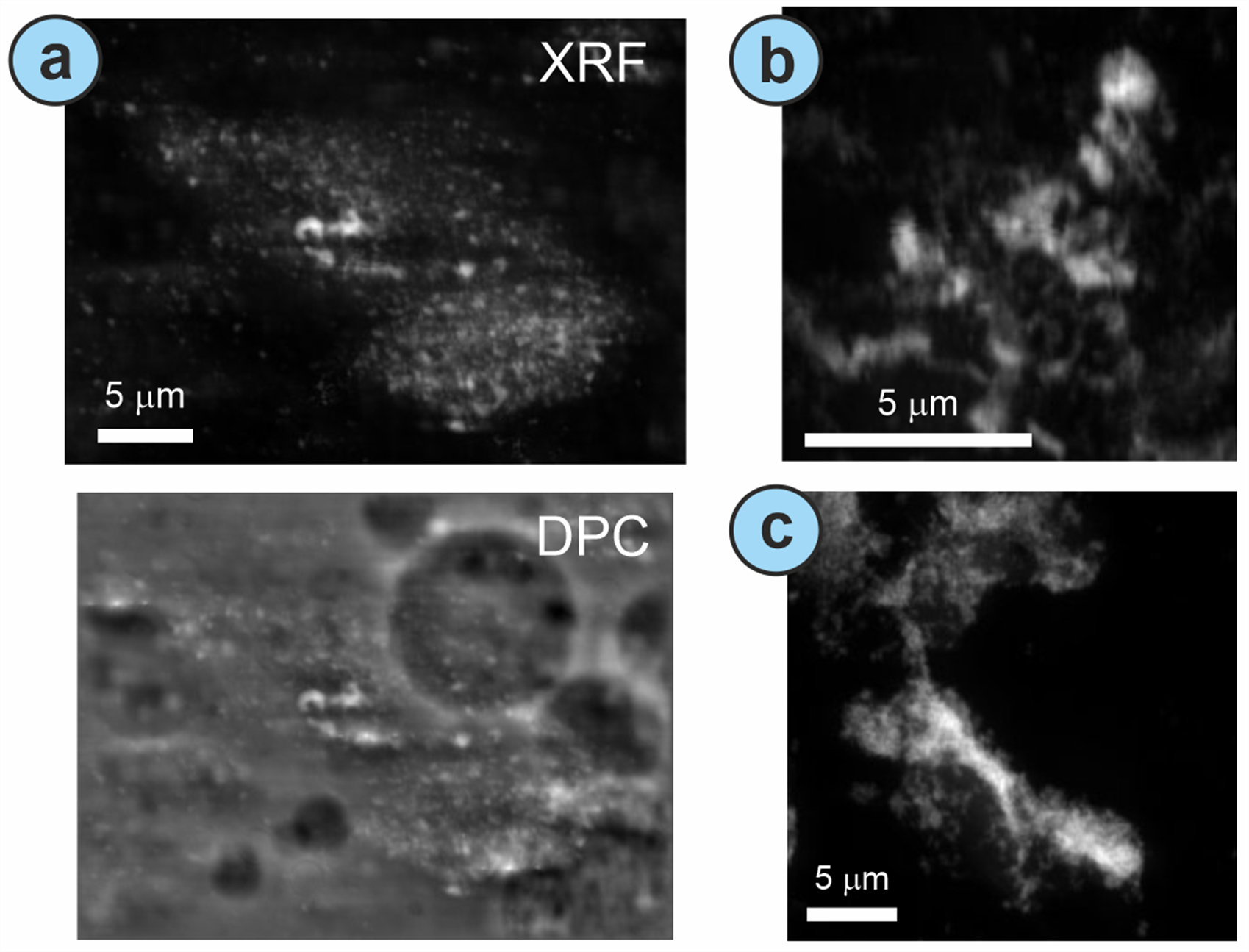
Figure 2.- a) X-ray fluorescence (XRF) image for the Zn K-edge (top) and differential phase contrast (DPC, bottom) for the Zn-particles deposited on polystyrene microplastics after incubating ZnO nanomaterials and MPs in tap water. b) XRF image revealing Zn-particles adsorbed to the polystyrene after leaching from the ZnO-based sunscreen aged in seawater. c) XRF image of ZnO nanomaterials adsorbed to irregularly shaped microplastics leached from the exfoliating cleanser incubated in seawater. © 2023 The Authors. Global Challenges published by Wiley-VCH GmbH. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited: Gomez-Gonzalez et al. Global Challenges. 2023, 2300036 (DOI: 10.1002/gch2.202300036).
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.