The Bradbury Lab at King’s College London, headed by Professor Elizabeth Bradbury, investigates damage to the central nervous system (CNS), and how the body responds to it. The traditional way of investigating soft tissue samples such as those of the central nervous system is 2D histology, in which slices are taken, stained and imaged. However, this process has limitations – slice thickness has a lower limit and measurements within cut slices are subject to inaccuracies arising from mechanical processing distortions. The group sent PhD student (now Dr) Merrick Strotton to the Diamond-Manchester Imaging Branchline I13-2 to investigate whether X-ray microtomography (a nominally non-destructive technique for taking a series of 2D images and turning them into a 3D volume) could avoid these issues. It wasn’t clear how to achieve the best possible results, and so alongside the biomedical studies, Dr Strotton worked with Diamond’s Dr Andrew Bodey on a series of methodological investigations on how to optimise imaging for soft tissue samples, the first results of which have recently been published in Scientific Reports.

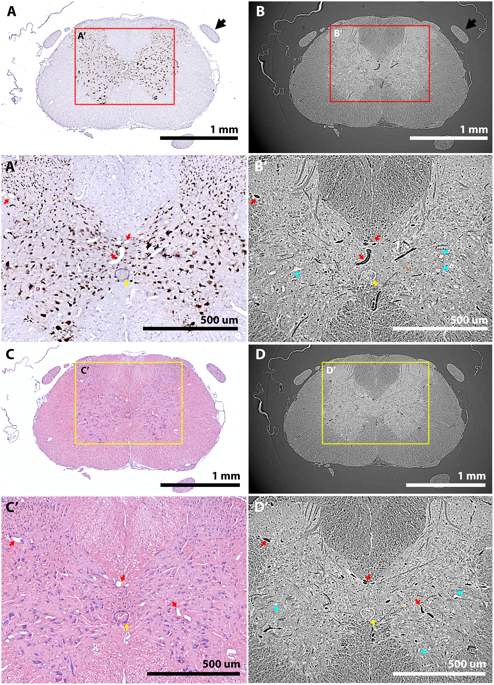
The CNS is a highly organised, 3D structure consisting of multiple elements that span the brain and spinal cord. 3D imaging enables these anatomical features to be studied in their full, spatial context, a considerable advantage over 2D histological studies. Synchrotron radiation X-ray microtomography (SRμCT) is a rapid and non-destructive technique, offering high spatial resolution, and able to image large (mm3) tissue regions with a large imaging depth and minimal tissue preparation. However, there are several challenges to carrying out X-ray microtomography on soft tissues, such as those of the CNS. Firstly, soft tissues give poor contrast, which can be improved using a variety of staining and/or optical techniques. Secondly, soft tissue is flexible, leading to issues with movement degrading achievable resolution. This is often handled by embedding samples in a more rigid medium, but this in itself can lead to problems, including the medium cracking in the beam. SRμCT does not currently allow specific labelling, so it lacks the ability of traditional 2D histology to discriminate finer tissue features (e.g. cell subtypes and subcellular markers). This limitation could be overcome by its use in combination with subsequent 2D histology, but many of the staining and embedding techniques used to prepare samples are irreversible, and render the samples unusable for histology studies.
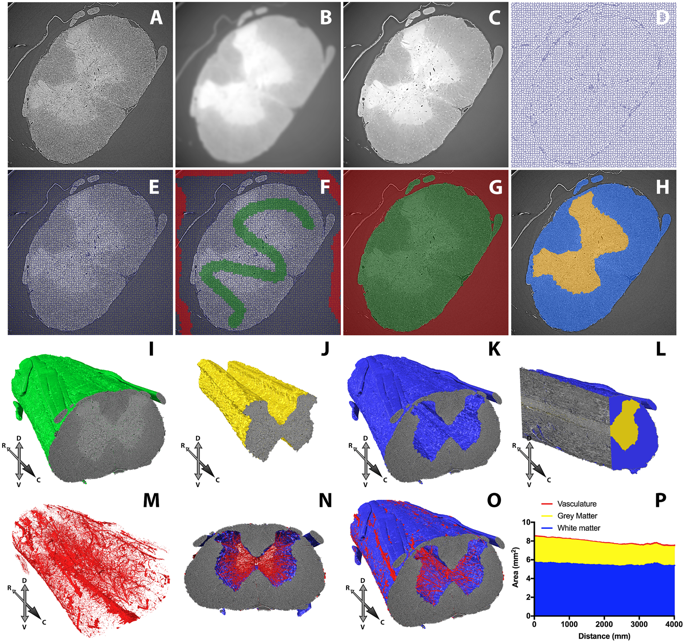
After investigating the common options for preparing and mounting CNS samples, the researchers determined that wax and epoxy resin had optimal mounting properties for SRμCT imaging. These choices also physically protect the samples during handling, and allow them to be stored at room temperature for long periods of time - meaning they can be prepared in advance of scheduled beamtime. Both wax and epoxy are cheap and easy to use; epoxy resin offers ultimate sample stability, but means the sample cannot then be used for histological studies. Wax embedding is reversible, and beam damage can be reduced to acceptable levels by undulator gap adjustment, high-pass filtration and minimising projection number to avoid over-exposing samples.
Phase contrast can be used to augment absorption contrast in soft tissue imaging. Inline phase contrast is a relatively straightforward phase imaging method in which contrast is increased by increasing the propagation distance between the sample and detector. The optimal distance is linked to the size of the features an investigator wishes to observe, and a balance must be found between the enhanced visualisation of features of interest and the resolution loss and segmentation difficulties that can arise. If tissue stains need to be avoided, then imaging with phase contrast alone is sufficient to reveal many soft tissue internal structures (albeit with less contrast).
Of the contrast-enhancing stains evaluated, Lugol’s iodine (LI) and osmium tetroxide (OsO4) provided the best tissue contrast. LI in combination with phase contrast enabled identification of capillary-level vasculature and large neuronal cell bodies, although it also led to some tissue shrinkage.
The number of images collected during tomography is a key determinant of data quality. Too few images results in a low signal-to-noise ratio (SNR) with poor contrast, but too many incurs a time penalty that may expose samples to unnecessary and damaging levels of X-radiation. Tests showed that increasing the number of images led to improved SNR and contrast. Contrast improvements peaked around 2,000 images, but SNR continued to improve. A sharp drop in image quality below 2,000 images indicated a minimum dataset; for routine work around 6,000 images is a good number.
Once the data have been collected, signal processing is needed to correct for image acquisition artefacts. These final steps include zinger artefact reduction, dark- and flat-field correction, lens radial distortion correction and ring artefact suppression. This article includes side-by-side comparisons to help assess the relative and cumulative merits of each step and inform future SRμCT studies of the CNS and soft tissues more generally.
The researchers used Diamond’s new tomography software Savu to make 3D reconstructions from 2D images. Savu is a cross-Diamond project, and the published paper includes acknowledgements to the people who wrote Savu and the modules used, information which Savu stores as part of the processing. New software from another Diamond project, SuRVoS, was used to segment features of interest from 3D reconstructions, with the aid of machine learning.
I13 is home to another innovation - the first of Diamond’s data beamlines. This includes a dedicated bank of high-powered workstations that users can book visits to (in a similar manner to X-ray beamline time) for data processing. According to Andrew Bodey, “The idea behind the data beamline was to offer computing power, suitable software and supporting expertise to users so that they can perform data analyses more efficiently than they may be able to at their home institutions. Visits are booked, from a day to a couple of weeks, giving users dedicated time to analyse their data, away from potential interruptions.” The data beamline is approaching its 100th visit, and Dr Strotton has made good use of it during his research.
By evaluating different sample preparations (embedding media, tissue stains), imaging parameters (projection number, propagation distance) and reconstruction parameters (artefact correction, phase retrieval), Merrick and Andrew have, together with other collaborators, developed a novel methodology (combining reversible iodine stain, wax embedding and inline phase contrast) for optimising fast, high-resolution complementary imaging of the CNS, which should be more broadly applicable to soft tissue samples in general.
As Andrew explains:
This paper should serve as a guide to help research groups optimise their soft tissue microtomography experiments with regards to staining, embedding, tomographic imaging, artefact correction and complementary imaging.
The researchers have applied their method to study spinal cord damage, and will publish the results soon.
To find out more about the I13 beamline, or to discuss potential applications, please contact Principal Beamline Scientist Christoph Rau: [email protected].
Strotton MC & Bodey AJ (joint first authors), Wanelik K, Darrow MC, Medina E, Hobbs C, Rau C, Bradbury EJ. Optimising complementary soft tissue synchrotron X-ray microtomography for reversibly-stained central nervous system samples. Scientific Reports 8, 12017 (2018). DOI: 10.1038/s41598-018-30520-8.
Bodey AJ, Rau C. Launch of the I13-2 data beamline at the Diamond Light Source synchrotron. Journal of Physics: Conference Series 849, 012038 (2017). DOI:10.1088/1742-6596/849/1/012038.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.