Chagas disease and leishmaniasis are two examples of Neglected Tropical Diseases (NTDs), a diverse group of 20 conditions that cause devastating health, social and economic consequences for over one billion people worldwide. Public health control of NTDs is challenging, as they are often related to environmental conditions and have complex epidemiology. Many have complex life cycles and are vector-borne, meaning that there are animal reservoirs. The World Health Organization's One Health initiative recognises that human and animal health are interlinked, and that an integrated, unifying approach is needed to balance and optimise the health of people, animals and the environment. In work recently published in Acta Crystallographica Section D: Structural Biology, researchers from Durham University and the University of Sao Paulo obtained high-resolution structures of cysteine synthase enzymes from the pathogens that cause Chagas disease and leishmaniasis and a related livestock parasite. Their results allow a greater understanding of cysteine synthase in these organisms, and give a starting point for the structure-based design of new drugs to treat these diseases.
Neglected Tropical Diseases (NTDs) are a group of infectious diseases that predominantly affect populations in low-income and tropical regions of the world. These diseases often afflict the poorest and most marginalised communities, leading to significant health and economic burdens and disproportionately affecting women and children. However, historically they have not received as much attention and funding for research and control efforts as other major infectious diseases.
Trypanosoma cruzi, which causes Chagas disease, and the 20+ species of Leishmania that cause leishmaniasis are trypanosomatid organisms. Another trypanosomatid, Trypanosoma theileri, infects livestock such as sheep and cattle. All of these parasites have a complex life cycle, involving both an insect vector and a mammalian host. They require trypanothione to survive in their host organisms, which they produce via cysteine biosynthesis. The chemical pathway involves converting O-acetyl-l-serine into l-cysteine via a reaction catalysed by cysteine synthase (CS).
The lead author of the study, Kate Sowerby explains
This enzyme is found in a lot of different organisms, but not in humans and other mammals, which makes it a very promising drug target. In order to explore those possibilities, we conducted biochemical and crystallographic studies of CS from T. cruzi (TcCS), L. infantum (LiCS) and T. theileri (TthCS).
The Durham group has BAG Access to Diamond, offering regular access to groups of users able to share beamtime. For this research, the team determined the crystal structures of the three enzymes using Macromolecular Crystallography (MX) in two different sessions: TcCS on I03 and LiCS and TthCS on I24.
Having access to the microfocus beamline I24 was particularly useful for TthCS, because my crystals were actually quite small," said Kate Sowerby. "We send our samples to Diamond and then log in remotely to control the data acquisition, and the setup for that is really wonderful.
Vector control is challenging for these parasites, due to the variety of reservoirs, resistance to insecticides and other factors. There is therefore a need for effective and affordable drug treatments. However, only two treatments are available for Chagas disease, both introduced in the 1960s. They cause severe side effects in 90% of patients, and the treatment completion rate is only 65%. An additional challenge is that they are less effective during the chronic phase of the disease, which is when most cases are diagnosed.
The main treatments for leishmaniasis are severely toxic and are most effectively administered in hospitals. With reports of increasing resistance to existing treatments, there is a great need for novel therapies.
The detailed high-resolution structures of the three enzymes produced during this work are a good first step towards that goal.
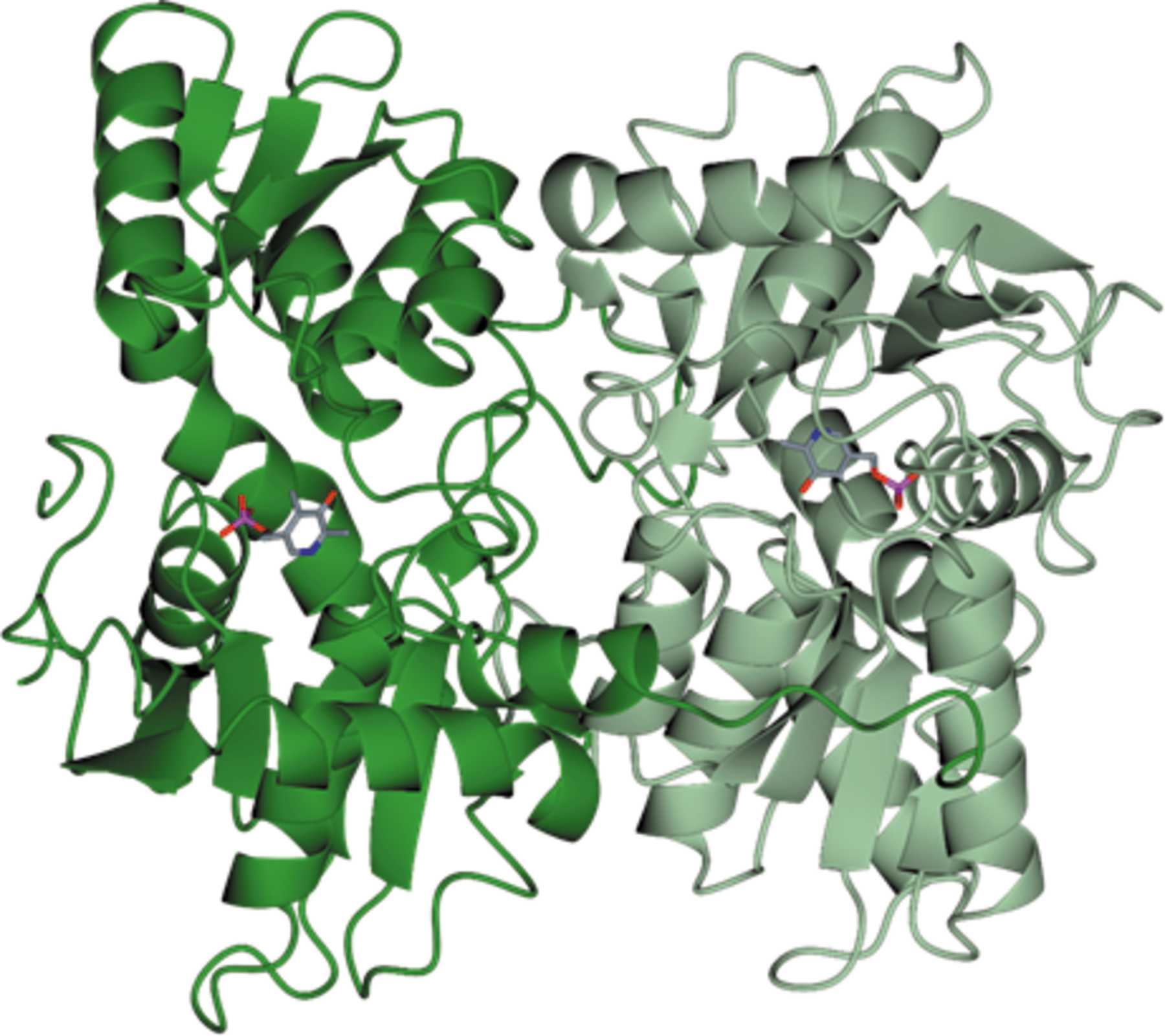
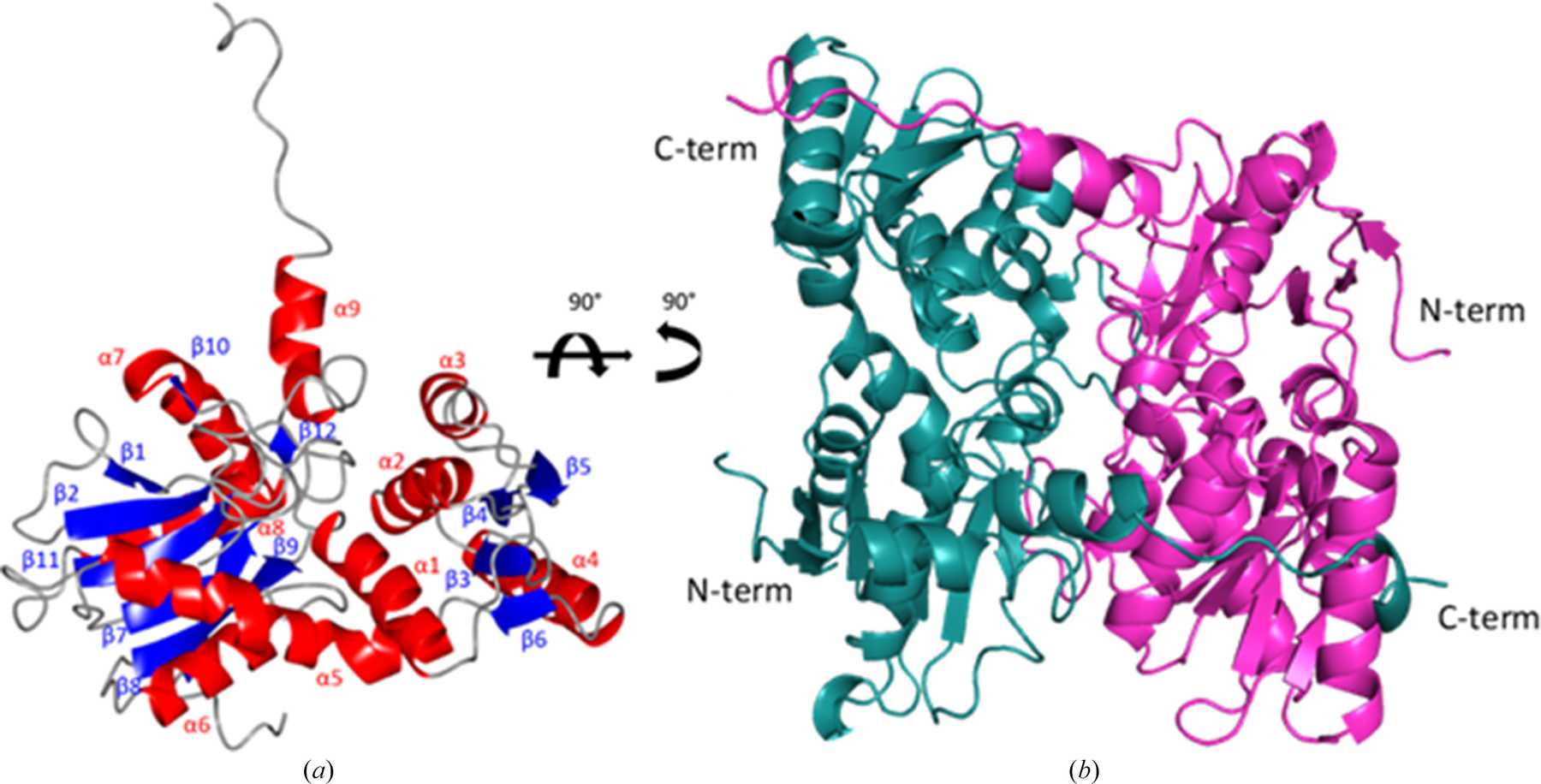
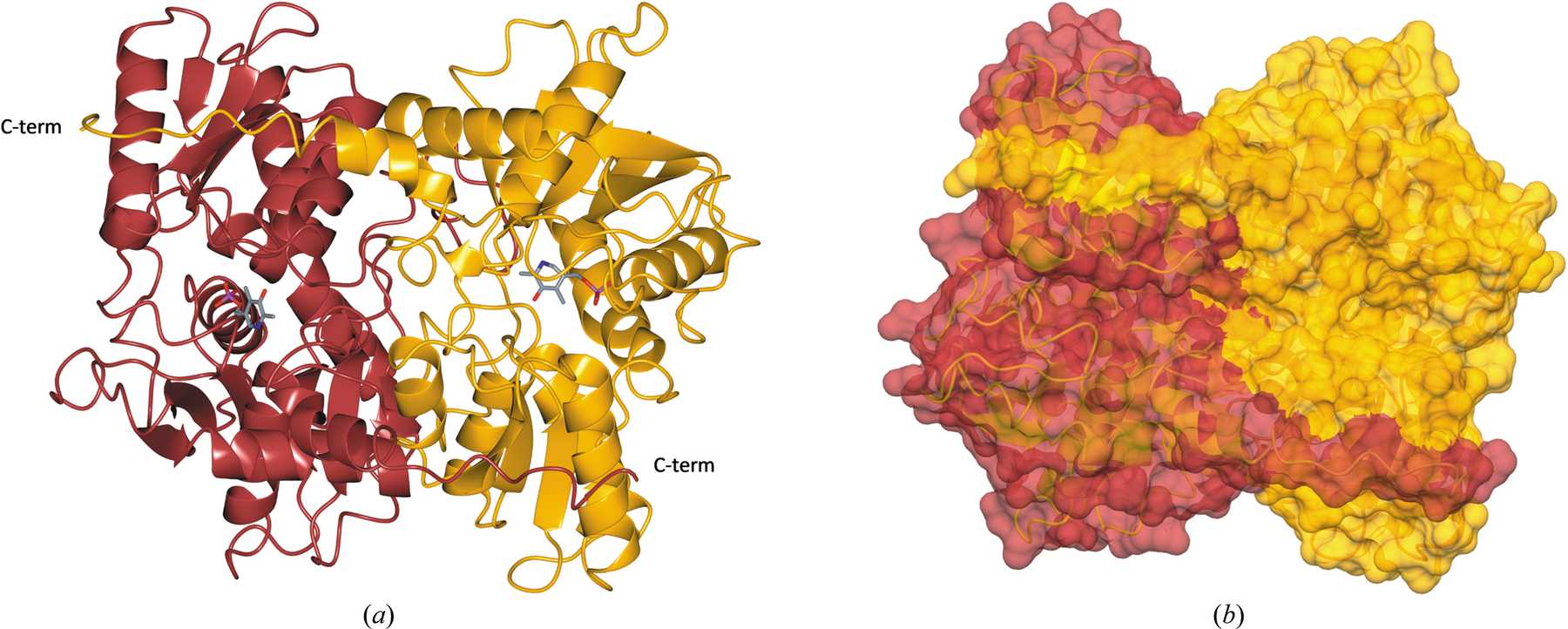
Kate Sowerby explains the next steps
I've been using these structures to do fragment-based drug design work. After some initial fragment screening, I've also recently used XChem to do a larger fragment search, with the ultimate goal of producing an inhibitor.
The team also has plans to test the first new compounds in the lab next year in Chagas Disease mouse models, with collaborator (and co-author) Prof Ariel Silber at the University of Sao Paulo.
Research like this is key to the World Health Organization achieving its goal to prevent, control, eliminate and eradicate neglected tropical diseases by 2030.
To find out more about the I24 beamline or discuss potential applications, please contact Principal Beamline Scientist Robin Owen: [email protected].
Sowerby K et al. Cysteine synthase: multiple structures of a key enzyme in cysteine synthesis and a potential drug target for Chagas disease and leishmaniasis. Acta Crystallographica Section D: Structural Biology 79.6 (2023). DOI:10.1107/S2059798323003613.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.