Emissions of radioactive iodine vapour pose a risk to workers in nuclear power stations, and widespread implementation of nuclear energy will require a solution. In a highly collaborative project, a team of researchers used a variety of techniques to investigate the ability of the MFM-300 series of robust porous metal-organic materials (MOFs) to adsorb iodine vapour. The results, published in the Journal of the American Chemical Society, showed good reversibility and an exceptional storage density, with the first experimental report of the self-aggregation of iodine into a triple helical structure, imaged at atomic resolution.
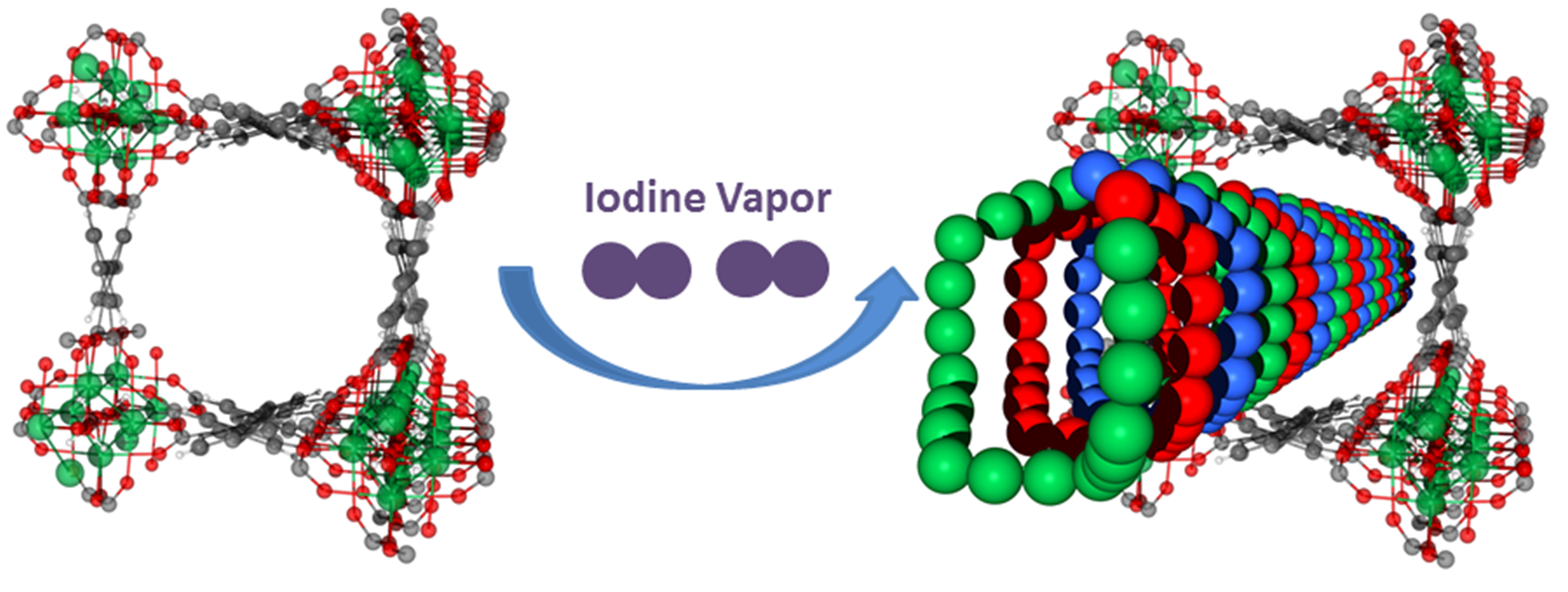
Figure 1: Iodine adsorption in a series of robust porous metal–organic materials, MFM-300(M) (M = Al, Sc, Fe, In).
Iodine storage in MOFs
Nuclear power supplied 11% of the world’s electricity in 2016. Nuclear fuel has an extremely high energy density, 500 million times higher than gasoline (by volume), but this comes with radiation hazards that must be carefully and strictly controlled. Opening a nuclear reactor (to remove spent fuel, for example) can cause a release of radioactive iodine vapour, which is hazardous to human health. A possible solution to this problem is to have a material outside the reactor (perhaps in the ventilation system) that can effectively capture the iodine vapour and prevent it from being released into to the atmosphere.
A practical system for iodine capture and storage will require high capacity and storage density (to minimise volume), stability and reversibility. Various systems have been investigated, but they generally suffer from irregular pore distributions, random adsorption sites and a lack of structural order, making it impossible for researchers to visualise interactions between the iodine and the host material, or between iodine molecules within the host pores.
Porous MOFs are being investigated as solid adsorbents for a wide range of molecules. They often exhibit high porosity and tuneable pore structures with desirable binding sites. However, studies of iodine adsorption in a number of MOFs show varying capacity, reversibility and stability. To design new materials, it is essential to understand the intermolecular contacts, location, and binding interactions of adsorbed guest molecules. This is a highly challenging task.
A unique, self-aggregating, triple helical structure
The original request for beamtime for this project was to carry out high-resolution X-ray powder diffraction (XRPD) on Diamond’s I11 beamline. Previous experiments investigating iodine adsorption in other systems suffered from poor crystallinity, limiting the amount of information that could be gathered. MOFs have very good crystallinity, allowing both neutron and X-ray studies. These experiments looked at iodine adsorption in a series of robust porous metal–organic materials, MFM-300(M) (M = Al, Sc, Fe or In), which show very high iodine adsorption.
Using I11 allowed the researchers to fully determine the position of the iodine molecules within the MOF pores, which are 0.7-0.8 nm in size. They found that when iodine approaches saturation inside MFM-300(Sc), it self-aggregates into an unusual triple helical structure, for which this is the first experimental report. The formation of these triple-helical chains allows the packing of iodine molecules to an exceptional density – 63% of that of solid iodine.
Rapid access and reversibility
Whilst at Diamond, discussions with beamline scientists brought up an idea for investigating the reversibility of iodine adsorption in MFM-300(M). An extra day of beamtime was granted via a Rapid Access request, and the researchers visited the MIRIAM: Infrared Microspectroscopy beamline (B22) to conduct far infrared imaging of the host-iodine interaction. The team were new to this kind of experiment, and found the assistance of the beamline scientists invaluable. The results showed that adsorption of iodine causes no notable distortion of the MOF system. The uptake of iodine within all MFM-300 materials is therefore reversible, and the MOF hosts can be fully regenerated via desorption under heating.
Dr Sihai Yang, from the University of Manchester, explained why this further experiment was necessary: “Reversibility is important in any commercial system, but there is typically a trade-off between the desire for strong interactions (to increase storage density) and the weak interactions that allow for reversibility. The MFM-300 materials show moderate interactions, offering both good storage density and reversibility.”
The insights gathered via this research will improved the development of future materials for iodine storage. Further work has been funded, based on these results, with a new PhD starting in September 2018. The aim is to investigate more MOF materials, with the expectation that this will uncover more complex iodine structures in different materials.
To find out more about the I11 beamline, or to discuss potential applications, please contact Principal Beamline Scientist Prof Chiu Tang: [email protected]. For B22, the Principal beamline Scientist is Dr Gianfelice Cinque: [email protected].
Zhang X et al. Confinement of Iodine Molecules into Triple-Helical Chains within Robust Metal–Organic Frameworks. Journal of the American Chemical Society (2017). DOI: 10.1021/jacs.7b08748
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.