HIV remains a major global health issue. In 2019, estimates suggested that 38 million people were living with the disease, and 690,000 people died of AIDS-related illnesses. HIV has a remarkable ability to mutate and evade conventional approaches to developing a vaccine. In work recently published in IUCrJ, an international team of researchers details a new strategy to fight the virus, which may ultimately provide the basis for active immunisation vaccines to stimulate an antibody response to block HIV infection.
Here, Prof Naomi Chayen, Dr Lata Govada and Dr Marc Morgan of Imperial College, Prof Emmanuel Saridakis of the Nanotechnology Institute Athens and Prof John Helliwell of the University of Manchester explain the importance of Diamond's role in their research.
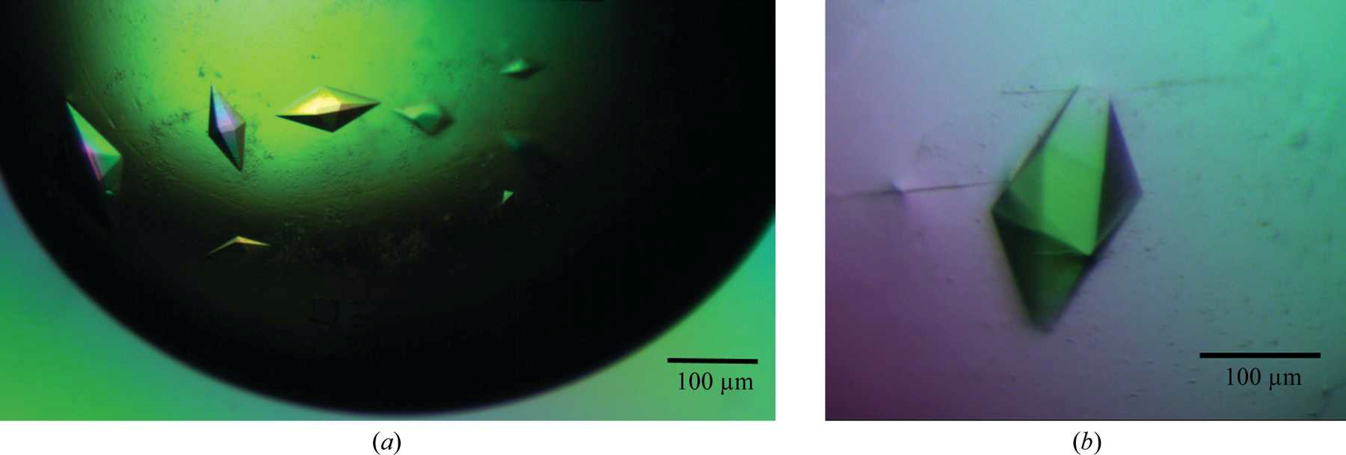
AIDS is a global pandemic with millions of people infected with HIV. The chemokine receptor CCR5 is one of the main entry co-receptors for HIV, and it needs to be blocked to prevent infection. It is, therefore, an important therapeutic target.
The aim of this project was to apply a new strategy to block CCR5 by designing synthetic peptides mimicking the part of CCR5 to which an antibody specific to it attaches itself (an epitope). This will stimulate the production of antibodies against CCR5. The most important part of the CCR5 for our purpose is the antibody-binding epitope in the N terminal domain. Prior research identifies PIYDIN as the core portion. Our immediate aim in this project was, therefore, to determine the structure of the epitope bound to a known anti-CCR5 antibody (RoAb13).
In short, the key insights into the 3D structures of the CCR5 N-terminal domain and how it binds to an anti-CCR5 antibody provide knowledge that will inform the design of better peptide analogues for use as immunogens in vivo.
Simply put, the overall aim of this research is to find a means of blocking the HIV infection (the chemokine receptor CCR5), with a view to the development of a successful, long-lived, low-toxicity method to prevent HIV infection.
The I04 beamline is a favourite among the user groups in Imperial College's Diamond BAG. The tuneable wavelength and microfocus capability make it an ideal beamline for experimental phasing and screening a wide variety of crystals. Since we collected the data featured in this publication, an Eiger detector has been installed at I04, making it even more attractive to our users.
For this particular data collection, Diamond's high-intensity monochromatic beam was important to this work. We chose X-ray diffraction as the most applicable probe of the structure of matter, versus Nuclear Magnetic Resonance (NMR) or neutrons or electrons, for the precise means of viewing the binding of the peptide to the antibody.
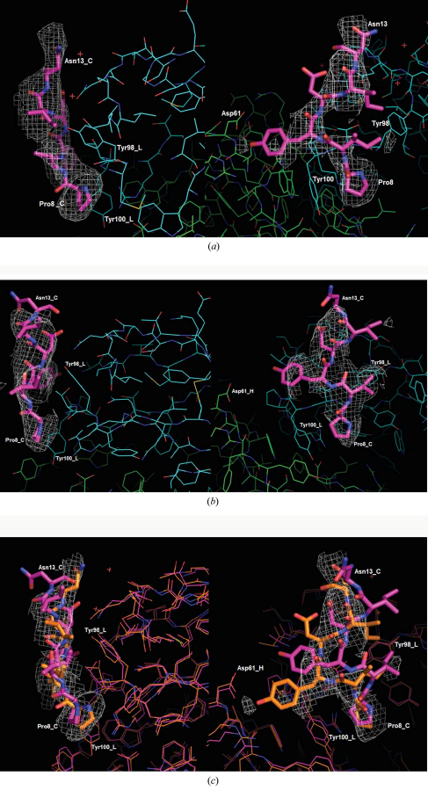
Synthetic peptides consisting of epitope sequences of the CCR5, and which bind to the antibody, were co-crystallised with the antibody. These antibody-peptide complex crystal structures showed us the antibody-epitope interactions.
In terms of our procedure in this research, we have determined two crystal structures. These show that pure PIYDIN binds to the antibody (PDB code 7nw3) and also very similarly when the PIYDIN is in the long (31 amino acids) peptide (PDB code 7njz). This polypeptide is the sequence in the ccr5 N terminal domain.
These two crystal structures taken together are an important demonstration of both the scientific reproducibility between the two and also their specific structure and function relevance.
The next steps will be to harness the structures which we have determined now, in order to seek an improved synthetic peptide binder. We will then determine this structure, i.e. with the synthetic peptide bound, and this will involve more experiments at Diamond. This improved peptide objective, thanks to its presumably better binding to the antibody, may well allow a higher resolution diffraction study
Diamond is certainly crucial to our research at Imperial College. Over 20 research groups at Imperial College regularly use Diamond as part of a BAG. Within the two year cycle of our BAG, Imperial College is typically allocated close to 80 sessions on the MX beamlines. In the last five years, we have screened nearly 13500 crystals at Diamond and collected over 4300 full datasets. This has resulted in the deposition of over 120 structures in the PDB and the publication of over 50 research papers in peer-reviewed journals.
We are very fortunate to have such a synchrotron facility at our disposal, and the wonderful beamline and administrative staff that we interact with most are always so accommodating. I (Marc Morgan) have been fortunate to use all of the MX beamlines as well as the XChem laboratory over some years now, and of course, I have formed friendships with some of the staff. I even know a couple of beamline scientists from the days when we were all at the Laboratory of Molecular Biophysics in Oxford, headed by Prof Dame Louise Johnson, who was also Director of Life Sciences at Diamond.
I am always amazed at how the MX beamlines are constantly upgraded with the user in mind, and this is only possible through great consultation between Diamond and its users. I am personally part of the XChem Superuser group, and as the main contact on the Imperial BAG, I am regularly consulted and asked to provide samples to test new features and capabilities on the beamlines.
In summary, we regularly and frequently use Diamond's beamlines, for which we are very grateful, and heartily thank all the staff at Diamond involved in establishing such smooth running measurements for us. In addition, we highlight the excellent data processing pipeline and the archiving of all the raw data at the Atlas data store of the Rutherford Appleton Laboratory, which we find invaluable for returning, sometimes multiple times as in this study, for reanalyses to optimise the final protein model.
John Helliwell was heavily involved in defining Diamond Light Source in the MX beamlines' scoping and overall, as CCLRC Director of Synchrotron Radiation Science. The latter included, formally, Chairing the Research Councils' Interface Committee to the Diamond Project Team in the early 2000s. Prior to that, he was the UK's representative to the European Synchrotron Radiation Facility (ESRF) Council for many years, as well as Chair of the ESRF Science Advisory Committee, and so the foundations of Diamond based on the ESRF as the first of the third generation SR sources, are embedded in many details of Diamond. John Helliwell has enthusiastically supported the Diamond II proposal in his recent Letter of Support for the future MX beamlines.
To find out more about the I04 beamline or discuss potential applications, please contact Principal Beamline Scientist Ralf Flaig: [email protected].
Govada L et al. X-ray crystallography studies of RoAb13 bound to PIYDIN, a part of the CCR5 N terminal domain. IUCrJ 8, 678–683 (2021). DOI: 10.1107/S2052252521005340.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.