The natural world is great at chemistry, and there are enzymes that can accomplish tasks that are very difficult for us to recreate in laboratories and industrial settings, such as turning cellulose into glucose for biofuels. Biomass (plant-based materials) are a renewable resource, and would be a useful feedstock for many chemical processes. When we try to scale-up the natural versions of these processes, however, we run into several problems: the reactions are slow, and normally only occur between 25-37°C. They also need water, and the need to purify the water makes them very energy-intensive processes. Research published in Nature Chemistry describes ground-breaking results from a team at Imperial College, London, who have achieved a 30-fold increase in efficiency in the glucosidase enzyme, using an ionic solvent rather than water.
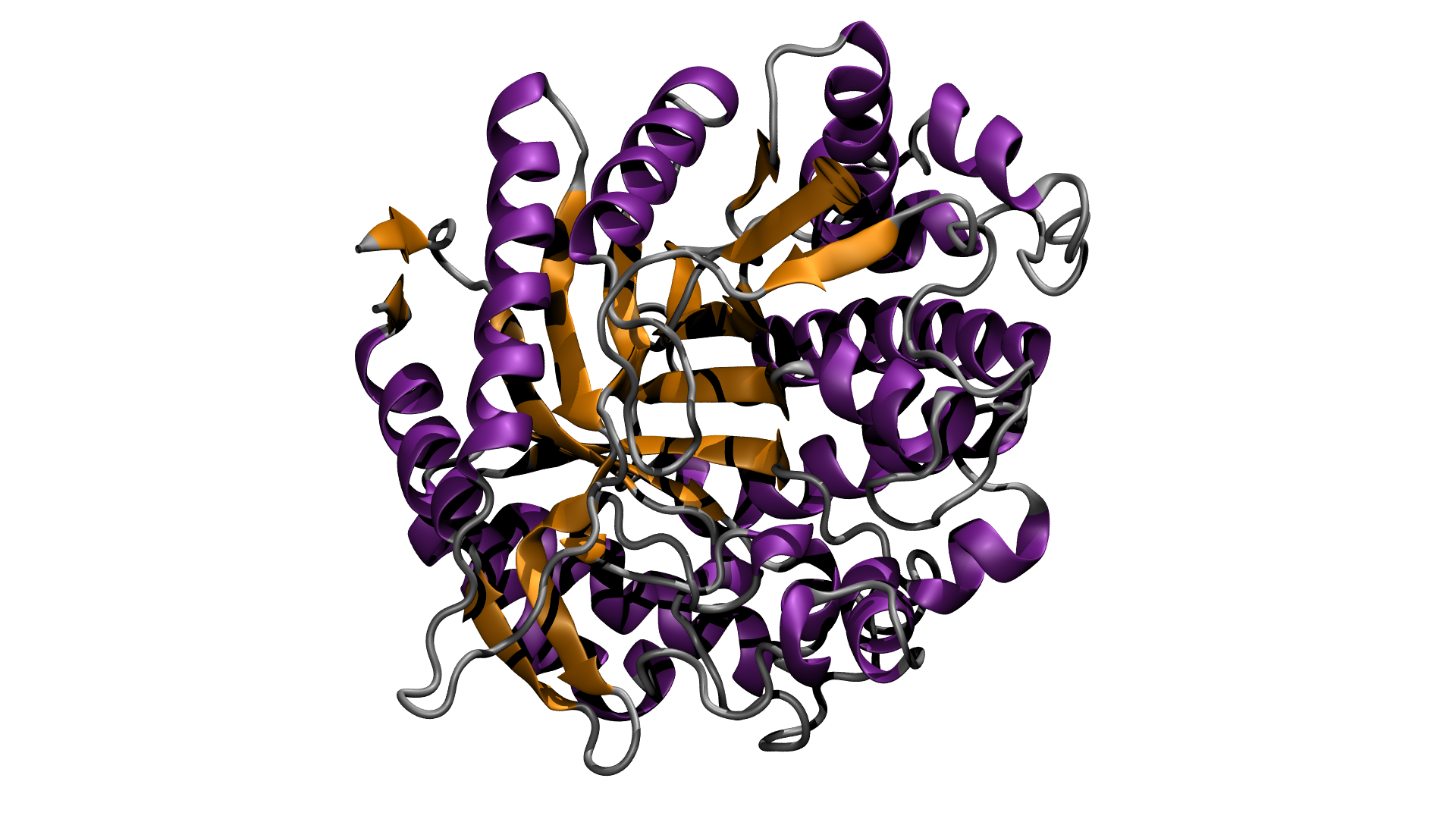
Industrial chemical systems struggle to break down cellulose into its component sugars, but there are enzymes in nature that easily accomplish this task. The problem with these biocatalysts is that they are difficult to incorporate into industrial processes, as they require large amounts of clean water, act slowly and only work in a very limited temperature range. A typical industrial process reducing cellulose to its component sugars relies on three different enzymes: two to make cellulose soluble in water, and a third to break it down. The third enzyme, glucosidase, is the bottleneck in the process, and the target of research to improve its efficiency.
Ionic liquids, salts in a liquid state, are a relatively new class of solvent that has promising industrial applications. Previous research has shown that biopolymers such as cellulose dissolve well in ionic liquids, and a research team from Imperial College, London, has been researching their use in improving the efficiency of glucosidase. They chemically modified a generic glucosidase from a common fungus, Aspergillus niger, to improve its stability in ionic liquids and at higher temperatures, then brought samples to Diamond to check that its enzymatic activity had not been impaired. Using synchrotron radiation circular dichroism (SRCD) spectroscopy on B23 allowed them to look at the enzyme’s secondary structure, showing that its characteristics are maintained in the absence of water and at high temperatures. B23 is unique in its ability to handle high temperature work and so was essential to this research that would have been unattainable with bench-top CD instruments and other worldwide SRCD beamlines. The researchers also used synchrotron radiation small angle scattering (SAXS) on I22, to check the 3D morphology (or shape) of the enzyme and demonstrate that it remained globular under processing conditions.
Lead author Dr Alex Brogan explains how the collaborative environment at Diamond is as important as the beamlines themselves: “Our group has been coming to Diamond for a number of years, and we collaborate with beamline staff to get the best possible results. Diamond staff are invaluable for helping to set up our complex experiments, solving an issues that arise, and assisting with the interpretation of results.”
Brogan APS et al. Non-aqueous homogenous biocatalytic conversion of polysaccharides in ionic liquids using chemically modified glucosidase. Nature Chemistry (2018). DOI 10.1038/s41557-018-0088-6
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.