Keep up to date with the latest research and developments from Diamond. Sign up for news on our scientific output, facility updates and plans for the future.
Proteins are large molecules essential to life; they perform vital functions such as repairing cells and tissues and producing enzymes for digestion. The structure of a protein determines how it functions.
Most proteins are built from two structural building blocks—the alpha helix and the beta strand. These can be combined in different ways to generate an amazing gallery of 3D shapes that give natural proteins their many functions.
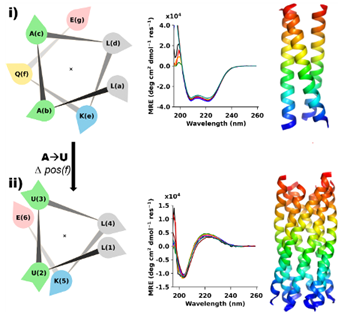
Over the decades biologists have been tweaking existing proteins to make them work better, or not at all, to assist and improve human health. Recently, scientists have been turning their attention to protein engineering – designing novel proteins to perform specific functions.
Using chemical synthesis, a team of scientists from the University of Bristol have designed a completely new molecule using a rare form of the alpha helix protein building block called the 3-10 helix. This was achieved by combining amino acids—the chemical units of proteins—that are found in natural proteins with others that are not
The team used Diamond’s X-rays to confirm their solution studies. Studying the crystallised form of the protein enabled them to see that the designer proteins formed bundles of pure 3-10 helices that could essentially act like a scaffold. This was a never-seen-before molecule.
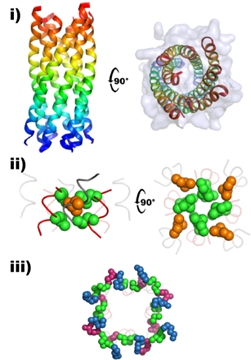
The creation of a novel, molecular scaffold opens up a range of possibilities with regards to designing proteins with a purpose. The Bristol team will now look to probe this so-called "dark matter" of protein structure to explore chemistries and functions that go beyond those of natural proteins.
Prasun K. et al. De novo design of discrete, stable 310-helix peptide assemblies. Nature (2022).
DOI: 10.1038/s41586-022-04868-x
Macromolecular crystallography beamlines: I03, I04, I04-1 and I24
Scientific disciplines: Life Sciences & Biotech; Biochemistry; Catalysis; Structural Biology
Technique: Macromolecular crystallography (MX)
BBSRC, EPSRC, European Research Council, Royal Society
Diamond press office: Isabelle Boscaro-Clarke, Head of Communications, [email protected]
X-ray crystallography: Neil Paterson, Beamline Scientist on I03, Diamond Light Source, [email protected] and Prasun Kumar, University of Bristol, [email protected]
Corresponding authors: Dek Woolfson, University of Bristol, [email protected] and Prasun Kumar, University of Bristol, [email protected]
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.