Our modern lifestyles can have detrimental effects on the environment, including producing an overabundance of carbon dioxide (CO2) that is fuelling climate change and a mountain of plastic waste that is proving challenging to recycle. Turning to green synthesis that uses CO2 and biomass as inputs allows us to make more sustainable plastics, suitable for low-energy recycling and safe disposal. However, these greener materials can struggle to match the properties of current petrochemical polymers. In work recently published in Advanced Materials, researchers from the University of Oxford describe new types of CO2-derived thermoplastic elastomers (TPEs) and investigate using a metal ion coordination strategy to augment tensile mechanical strength and Young's modulus. Their results show significant benefits to using low quantities of metals, suggesting that these materials have the potential to replace petrochemical polymers in high-growth fields such as medicine, robotics, and electronics.
Polymers are chemically bonded long chains of repeat units (monomers). Carbon dioxide is an attractive raw material - overabundant and cheap. With worldwide polymer production emitting 1.8 gigatonnes of CO2-equivalent every year, copolymerisation of CO2 with epoxides could become a useful carbon-capture and utilisation (CCU) technology, allowing existing manufacturing plants to turn waste products into sustainable polymers.
Lead author Kam Poon, a DPhil student at the University of Oxford says;
Carbon dioxide is a very attractive monomer for obvious reasons - it's abundant, it's cheap and it's a greenhouse gas. However, the plastics produced from CO2 and epoxides via ring-opening copolymerisation (ROCOP) tend to be brittle. We've found that combining them with a much softer and more flexible polymer gives us more control over their properties and produces superior materials.
The researchers developed block copolymers with ABA structures, combining CO2-derived poly(carbonates) (A-blocks) with poly(𝝐-decalactone) (B-blocks), 𝝐-decalactone being a soft and flexible monomer derived from castor oil. Changing the ratios of the two blocks gives TPEs with different properties. Mr Poon continues;
We went on to functionalise the polycarbonate block with carboxylate ligands - species that combine to metals - and investigated how adding small quantities of different metals tune those mechanical and thermal properties.
These kinds of biopolymer–metal networks occur in nature, strengthening materials that, for example, anchor mussels to rocks, harden insect mandibles and reinforce shells and bones.
Block polymer TPEs have phase-separated ABA structures, with rigid or glassy polymer blocks as the A domains and elastomers as the B domains. Mr Poon says;
Because the blocks themselves are immiscible, when we form these block copolymers they form micro phase-separated structures. Depending on the ratios of the blocks, and how immiscible they are, you typically see this phenomenon where the different blocks separate on a very small scale - domains of nm here. We could have spheres of one material in a matrix of the other block if that's the dominant block. And as you increase, you get these hexagonally-packed cylinders throughout the material, which is what we see in all cases in my materials in this paper. The domain has a large effect on the mechanical properties of the material.
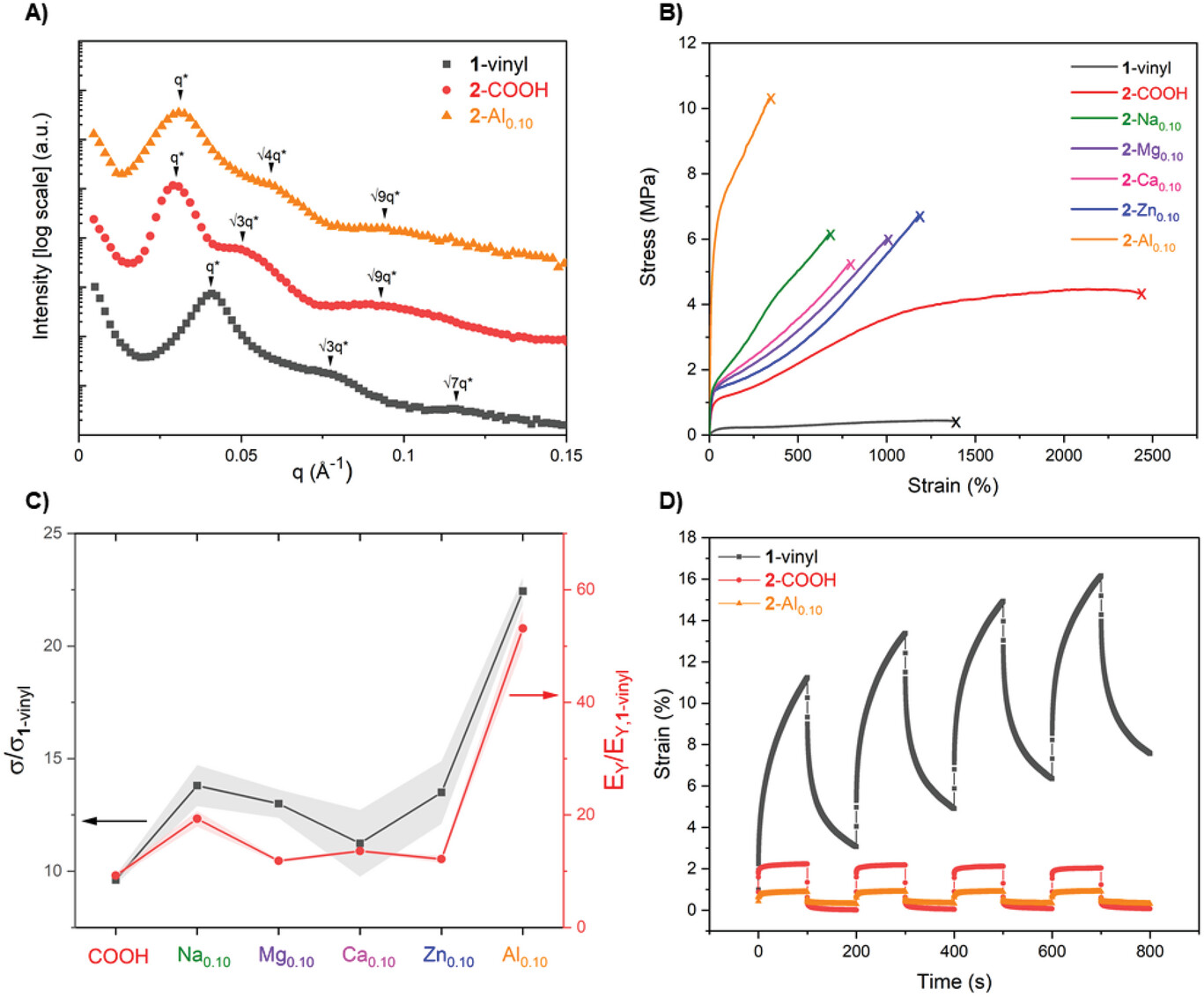
To understand the block polymer morphologies in the CO2-derived TPEs, the researchers conducted Small-Angle X-ray Scattering (SAXS) at room temperature, using the mail-in service available for labSAXS. Diamond's EPSRC funded offline facility offers Small- and Wide-Angle X-ray Scattering (SAXS/WAXS) for high-end characterisation of materials on the nanoscale and in situ and operando experiments under process and synthesis conditions.
The results showed that all the TPEs exhibited hexagonally packed cylindrical morphologies, allowing comparisons of the influences of different metals without altering their phase-separated microstructures. The team was then able to move on to mechanical testing of the TPEs, assess the stress-strain relationships and explore how the mechanical properties changed with metal–carboxylate coordination chemistry.
This work shows that installing metal-carboxylate ligands in CO2- and bio-derived TPEs offers control over and improvements to tensile mechanical properties. The resulting materials had wide operating and processing temperature ranges and good environmental stability and were mechanically recyclable. Mr Poon concludes;
Although we can design polymers from the ground up to have certain properties, scaling them up to pilot or commercial plants often requires re-optimisation of production conditions. These new TPEs offer the potential for using one master polymer and tuning it for different applications by adding a small amount of different metals, without the need for new infrastructure.
To find out more about the labSAXS facility or discuss potential applications, please contact Principal Beamline Scientist Nick Terrill: [email protected].
Poon, KC et al. Toughening CO2‐Derived Copolymer Elastomers Through Ionomer Networking. Advanced Materials (2023): 2302825. DOI:10.1002/adma.202302825.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.