The growing concern over air pollution caused by sulphur dioxide has driven the search for new and efficient methods to capture and remove it from industrial emissions and to enable its recovery from exhaust gases and conversion into chemical feedstocks. However, the highly corrosive and reactive nature of sulphur dioxide generally leads to severe structural degradation in capture materials.
Metal-organic frameworks are porous, crystalline materials, and zirconium-based metal-organic frameworks (Zr-MOFs) have emerged as promising materials for sulphur dioxide capture due to their high surface area and tuneable pore environment. Therefore, understanding the interactions between sulphur dioxide and the pore environment of Zr-MOFs is essential to designing efficient new materials for sulphur dioxide capture.
By studying the pore environment and its impact on sulphur dioxide adsorption, researchers can develop new principles for the design of MOFs with high sulphur dioxide adsorption at both low and high concentrations, enabling their use in a broader range of applications and contributing to the mitigation of air pollution. In addition, the development of regenerable methods for sulphur dioxide capture and recycling of the sorbent material can also reduce waste production and support the sustainability of the technology.
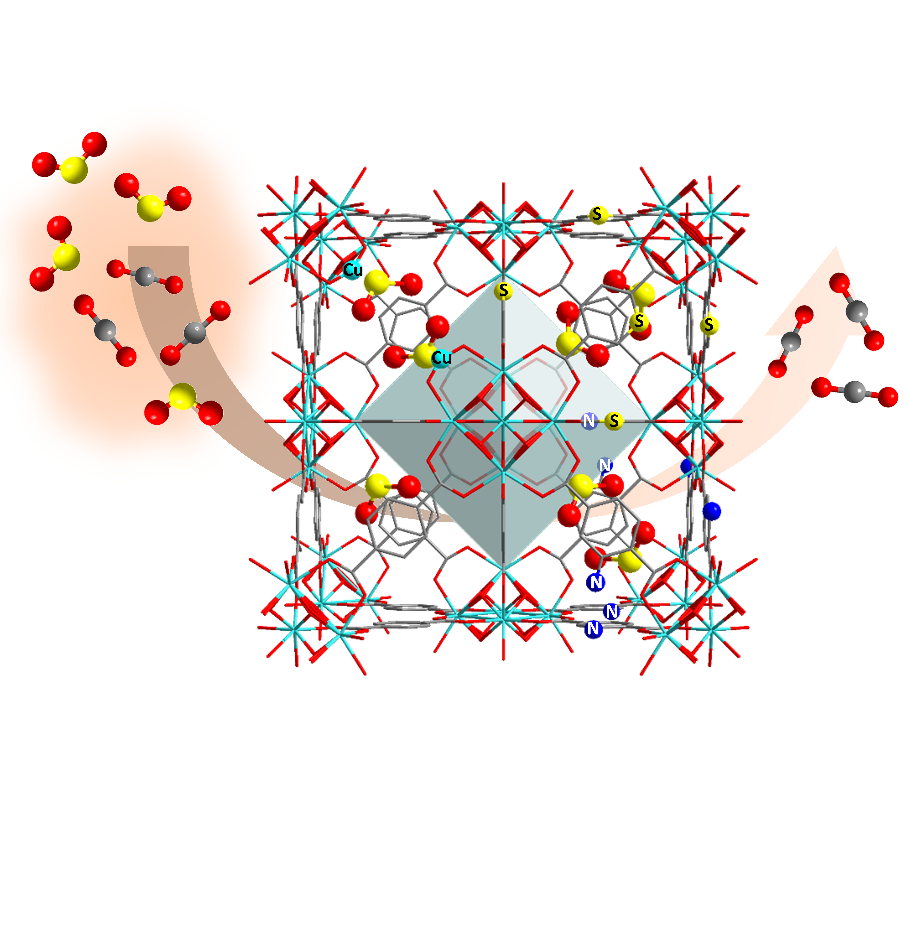
The porous nature of MOFs allows them to capture guest molecules, and host-guest interactions are of fundamental importance. Researchers from the University of Manchester used a combination of infrared micro-spectroscopy on Diamond's B22 beamline and in situ X-ray diffraction on I11, together with inelastic neutron scattering at the ISIS Neutron & Muon Source to enable the visualisation of the binding domains of adsorbed sulphur dioxide molecules and host-guest binding dynamics in Zr-MOFs at the atomic level.
Their results demonstrated that introducing functional groups (i.e. -NH2 and –S-) and atomically-dispersed CuII sites into a family of Zr-MOFs can effectively enhance the adsorption of sulphur dioxide at low pressure. In addition, the confined metal-ligand cages in Zr-bptc offer an optimal pore environment for effective sulphur dioxide capture and conversion.
Revealing the role of the pore environment (including pore size, pore geometry and functional groups) and understanding the fundamental host-guest chemistry at the atomic scale is making a revolutionary change to the design of the next generation of materials.
This work will inform the design of new materials optimised for sulphur dioxide capture. These could be applied to storage systems to minimise transport costs and space. In addition, materials with exceptional sulphur dioxide capture capability at low pressure will incubate the development of in-vehicle desulphurisation devices.
Li, J. et al. Structural and dynamic analysis of sulphur dioxide adsorption in a series of zirconium‐based Metal-Organic Frameworks. Angewandte Chemie International Edition 61,36: e202207259 (2022). DOI:10.1002/anie.202207259
EPSRC, grant number EP/I011870.
ERC, grant agreement No 742401, NANOCHEM.
Martin Schröder, Department of Chemistry, University of Manchester, [email protected]
Sihai Yang, Department of Chemistry, University of Manchester [email protected]
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.