Organic electronic devices, based on organic materials instead of traditional inorganic semiconductors, are already finding their way into consumer devices. Your smartphone display, for example, may take advantage of organic light-emitting diodes (OLEDs). Organic electronics is a rapidly growing field of research developing next-generation electronic devices with potential applications in everything from healthcare to renewable energy. The unique properties of organic materials, such as their flexibility, low cost, and environmental sustainability, make them an attractive alternative to traditional electronic materials. However, although printable optoelectronics show great promise in the lab, this is frequently not transferred to real-world situations. The problem lies in the molecules' tendency to stick together, an aggregation that adversely affects their electronic properties. In work recently published in Angewandte Chemie, an international team of researchers report the development of a self-assembling supramolecular pseudo-cube formed from six perylene diimides (PDIs). This rigid cage assembly prevents these chromophores from aggregating and therefore retains their electronic properties. It also creates an excited multimer that acts as an excited-state reservoir. Their results demonstrate that self-assembly is a powerful tool for retaining and controlling the electronic properties of organic semiconductors, bringing molecular electronics devices within reach.
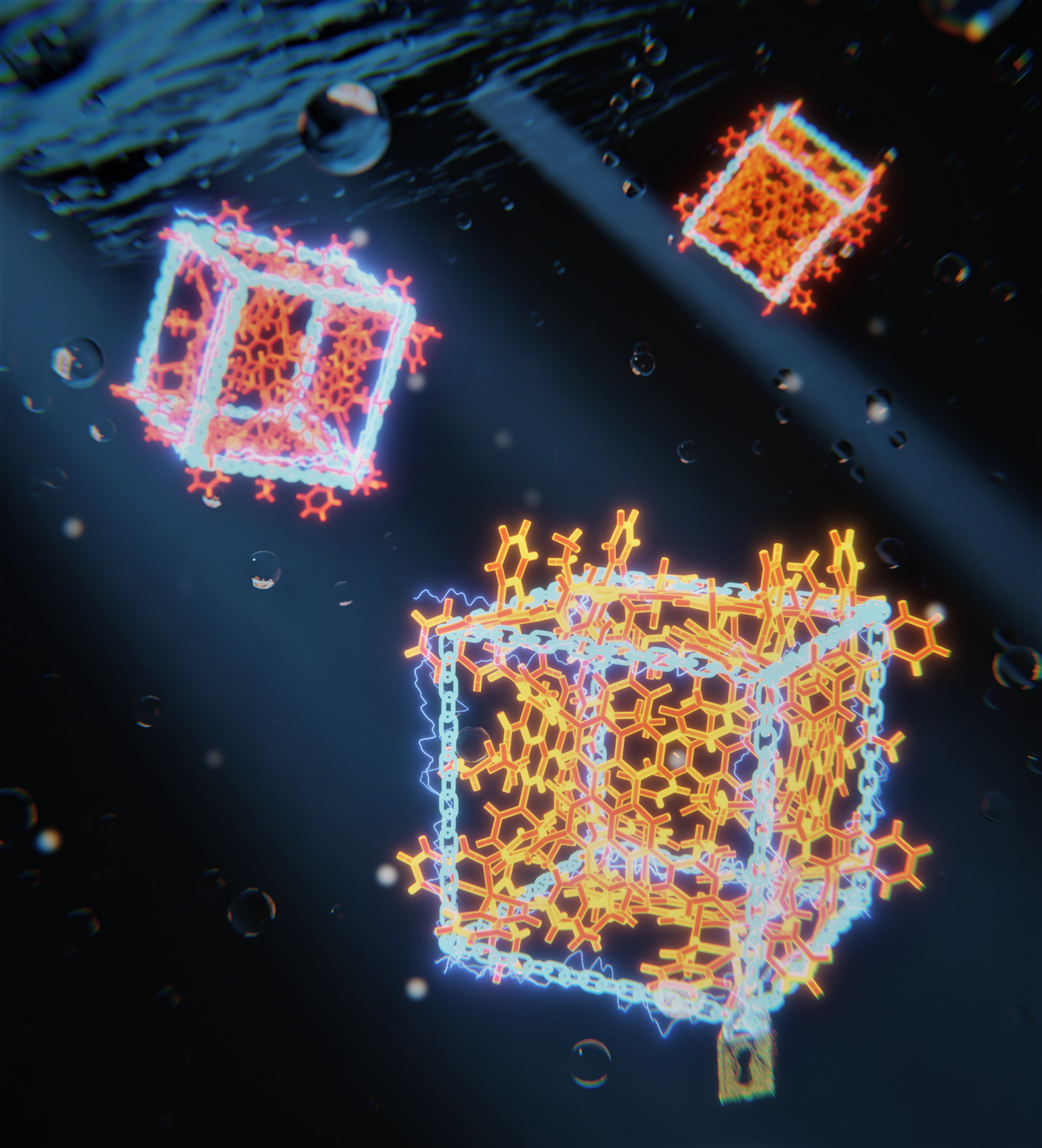
Chromophores - molecules that absorb and emit light - can be used in optoelectronic devices that rely on the efficient conversion of light into electrical energy and vice versa, such as organic light-emitting diodes and solar cells. Examples of π-conjugated chromophores are found everywhere, including fabric dyes and the photoactive molecules plants use for photosynthesis. Chromophores are now the subject of research investigating their optoelectronic properties, and their potential for use in molecular electronics applications, such as organic light-emitting diodes (OLEDs) and organic photovoltaics (OPV).
Perylene diimide (PDI) is one example, a chromophore often used in pigments and organic electronics. However, although PDI and similar molecules show excellent optoelectronic properties individually, the picture becomes more complex when they are manufactured into thin films for use in devices. In thin films, the chromophores tend to aggregate, leading to excited-state quenching that reduces their efficiency.
Dr Sascha Feldmann from Harvard University said:
I've always been very interested in the work of Jonathan Nitschke and his group at the University of Cambridge, developing self-assembling supramolecular cage-like structures, and this was a fantastic collaboration to see whether building a supramolecular cage of chromophores can prevent the molecules from aggregating and quenching, and so retain their optoelectronic properties in thin films.
The first step was synthesising a suitably modified building block, based on perylene diimide, with additional phenylamine and neopentyl groups to provide sites for linkage and enhance solubility. During the subsequent supramolecular self-assembly, the building blocks reacted with zinc(II) ions and picolinal to build the rigid cube structure.
That was the goal, but had it worked? Dr Feldmann explained:
No matter how beautifully you simulate these structures on the compute. Ultimately, you need proof that your clever design of these molecules worked out and the linkers really did self-assemble into the supramolecular cube. And that's where Diamond comes in, because we need a single-crystal X-ray diffraction (XRD) pattern to prove we have created the desired structure. The larger these supramolecular cages are, the harder it is to get an XRD structure. Dr Tanya Ronson and PhD students Zifei Lu and Ina Heckelmann successfully got that pattern at I19, and the cube was as we designed it. It looked fantastic.
With proof that the supramolecular cube was correct, the Feldmann group could use their speciality - ultra-fast spectroscopy - to evaluate its optoelectronic properties. Their results showed that - confined to the rigid cage structure - the chromophores could not aggregate and retained their optoelectronic properties.
There was a second, unexpected effect of the cube.
Dr Feldmann said:
We found proof of something we coined an excited multimer state, derived from the concept of an excimer. An excimer is an excited-state dimer, implying two chromophores. In this case, we cannot directly determine the exact number of coupled chromophores in our system. It gives rise to an additional, longer lived, red-shifted broad emission that seems to act as an excited state reservoir.
Exciting as these discoveries are, the team has a long list of future research projects, including making the molecules chiral to see if they can produce circular-polarised light and introducing guest molecules into the supramolecular cages.
It looks like printable optoelectronics have a very bright future ahead.
To find out more about the I19 beamline or discuss potential applications, please contact Principal Beamline Scientist Dave Allan: [email protected].
Heckelmann, I et al. Supramolecular Self‐Assembly as a Tool to Preserve Electronic Purity of Perylene Diimide Chromophores. Angewandte Chemie International Edition (2022). DOI:10.1002/anie.202216729.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.