Coeliac disease (CoD) is an autoimmune condition triggered by the gluten found in wheat, barley and rye - three cereals common in the Western diet. To avoid severe symptoms that reduce their life expectancy, patients must stick to a strict gluten-free diet throughout their lives. Therefore, researchers are investigating whether it's possible to develop an oral enzyme therapy to alleviate the problem. In work recently published in Nature Communications, a team led by Dr F. Xavier Gomis-Rüth from the Institute of Molecular Biology of Barcelona in Catalonia has been studying neprosin, an enzyme initially extracted from the digestive juices of a carnivorous plant. They used X-ray diffraction data collected at Diamond to determine the 3D structure of neprosin, showing it to be a zymogen only active at gastric pH. Their results suggest that neprosin is a very effective glutenase and could ultimately lead to a CoD therapy.
Coeliac disease (CoD) is a common autoimmune condition affecting at least 1 in 100 people in the UK. The disease is caused by an adverse reaction to gluten, a protein found in wheat, barley and rye, and damages the small intestine, affecting the uptake of nutrients. Intestinal damage can occur after eating as little as 10 mg of dietary gluten in a day, which is less than 0.1% of the amount found in a typical Western diet. There is currently no treatment for CoD other than avoiding foods that contain gluten. In recent years this has become easier, with a variety of gluten-free products becoming available. However, these tend to be more expensive than ordinary food products and can be less palatable due to different textures and flavours. Achieving balanced nutrition is also challenging on a gluten-free diet.
"Gluten" is an umbrella term referring to the combination of glutelin and prolamin proteins that store nutrients in plants. The problem lies in the way the human digestive system deals with gluten, breaking it down into smaller peptides that can be toxic. The 33-mer peptide, for example, is a fragment of alpha gliadin, a prolamin in wheat. In people with coeliac disease, 33-mer strongly binds to a receptor in the immune system, triggering an autoimmune response.
There is, therefore, a need for effective CoD therapies. People with lactose intolerance can take lactase tablets, an oral enzyme therapy that breaks down the lactose they find indigestible. Researchers are investigating a similar approach to treating CoD, and whether it is possible to develop a so-called 'glutenase' that could break down toxic peptides. This kind of enzyme therapy would also help people with irritable bowel syndrome and non-coeliac gluten sensitivity. However, to be effective, a glutenase must efficiently work in the stomach, at a pH of 2-2.5, without itself being broken down. To avoid damaging the intestines and inhibiting nutrient absorption, the glutenase would then ideally be inactive at the higher pH in the intestines. Finally, it has to be effective at a reasonable dose.
Previous research has identified potential candidates in insects, fungi, bacteria and germinating cereal grains. However, even the best of these are insufficiently active at low pH, or require very high doses or protective modifications such as PEGylation or microencapsulation. None has yet demonstrated an ability to replace a gluten-free diet.
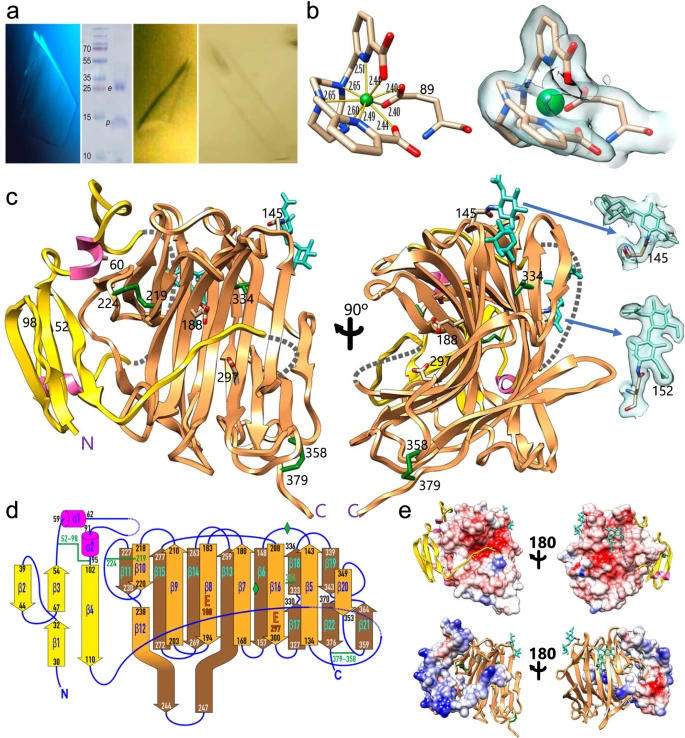
In this research, based on previous pioneering studies by the group of Dr David Schriemer from the University of Calgary in Canada, scientists at the Molecular Biology Institute of Barcelona in Catalonia have shown that an enzyme (neprosin) found in the digestive juices of a pitcher plant (Nepenthes x ventrata) can dismantle the toxic peptides.
Previous studies illustrated the potential of neprosin. However, producing enough of the enzyme by purifying pitcher plant extracts was not feasible.
"Our major breakthrough was to develop a eukaryotic expression system using human cells," says Dr F. Xavier Gomis-Rüth." This human recombinant production system produces high yields of neprosin for our studies."
The team sent neprosin crystals to Diamond, using remote-access X-ray diffraction on beamline I04-1 to resolve the 3D structure of the enzyme. The synchrotron data revealed the overall and active-site architecture of neprosin and its catalytic mechanism, and showed it to be a glutamate peptidase, a rare class of peptidases. Moreover, the results show that neprosin is a zymogen, an inactive substance converted into an active enzyme in the low pH conditions found in the stomach.
"We found that, in vitro, neprosin quickly and effectively breaks down gliadin and 33-mer, and that it becomes inactive at the higher pH found in the intestines," Dr Gomis-Rüth explained. "Furthermore, we've shown that a reasonable dose can reduce the amount of the toxic peptide epitopes in the small intestine of a mouse model by up to 90%, which is a very substantial reduction in the protein's ability to trigger a CoD response."
While sticking to a gluten-free diet remains the only option for CoD sufferers, this work shows that a pitcher plant has provided a promising candidate for a bona fide glutenase, and some hope of an effective CoD therapy in the future.
To find out more about the I04-1 beamline or discuss potential applications, please contact Principal Beamline Scientist Frank von Delft: [email protected].
del Amo-Maestro L et al. Molecular and in vivo studies of a glutamate-class prolyl-endopeptidase for coeliac disease therapy. Nature Communications 13.1: 4446 (2022). DOI:10.1038/s41467-022-32215-1.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.