Dopamine is a type of chemical messenger (neurotransmitter) which plays a vital role in the central nervous system, involved in numerous essential bodily functions, including controlling our mood, memory, sleep, and movement. The tyrosine hydroxylase enzyme is critical to the biosynthesis of dopamine in the brain. Deficiency or malfunction of tyrosine hydroxylase (TH) results in a depletion of dopamine, causing Parkinson’s-related disorders (Parkinsonisms). On the other hand, too much dopamine is also harmful to brain cells, so maintaining an appropriate balance is key to normal brain function. Due to a lack of detailed information on the structure of full-length human TH, understanding of its interaction with dopamine and the role of this interaction in regulating TH activity is missing. In work recently published in Nature Communications, an international team of researchers led by Professors José Valpuesta (Centro Nacional de Biotecnología. Madrid) and Aurora Martínez (Bergen University) employed state-of-the-art cryo-electron microscopy facilities at eBIC, Diamond’s electron Bio-Imaging Centre, centre to solve the structure of full-length human TH for the first time. Their results also demonstrate the essential molecular processes behind inhibition and activation of TH.
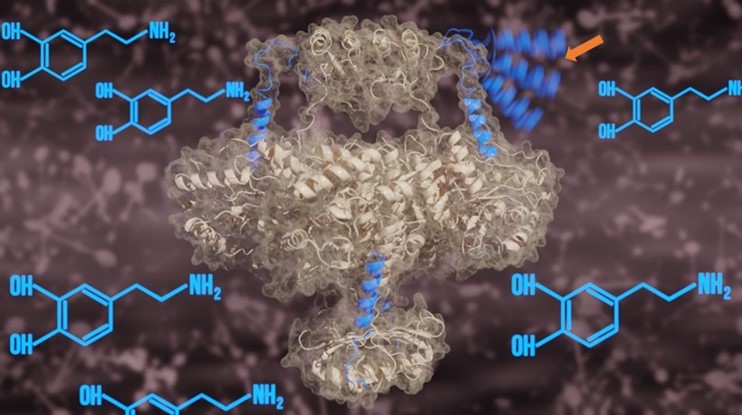
TH plays a pivotal role in the conversion of amino acid tyrosine into dopamine. It is known that dopamine interaction with TH inhibits the activity of TH, whilst phosphorylation at specific site reactivates the enzyme. However, understanding precisely how this regulatory mechanism functions at the molecular level requires a deeper understanding of the structure of full-length TH. This is key to developing therapies to target malfunctioning TH in Parkinsonisms. The structure of full-length TH had been elusive until now, remaining an analytical challenge.
Professor José Valpuesta explains:
The TH complex is very flexible, inherent to its function. This makes TH difficult to crystallise for analysis by X-ray crystallography. The whole complex is also too big to analyse by nuclear magnetic resonance (NMR).
Cryo-electron microscopy (cryo-EM) enables researchers to study proteins at atomic or near-atomic spatial resolutions, whilst also keeping the sample in a frozen-hydrated state. This minimises any chemical or structural changes to the sample which may occur during preparation. The latest advancements in cryo-EM are enabling researchers to significantly improve data quality.
Professor Valpuesta explains:
The most important aspect of the cryo-EM revolution is the advancements in the efficiency and speed of modern electron detectors. It is now possible to acquire several hundred images per second. This is very important because when the sample is irradiated by the electron beam, thermal energy deposited in the sample causes it to move. This negatively impacts spatial resolution. By cross-correlating hundreds of images, the spatial resolution required to view single molecules can be restored.
In this work, the team utilised the 300 kV Titan Krios cryo-electron microscope at Diamond’s eBIC facility to analyse the structure of recombinant human TH. The central structure of TH was shown to be formed from four sub-units (tetramer) with regulatory and catalytic domains (distinct functional regions of the enzyme structure) separated by a distance of 1.5 nanometres. This distance is approximately 200,000 times smaller than the diameter of a grain of salt!
The team also investigated differences in the structure of TH in the presence and absence of dopamine, noting some interesting changes at the enzyme’s active site. When in the presence of dopamine, an alpha helix protein structure, detached from the main TH complex in the absence of dopamine, was shown to penetrate the active site of TH. This enabled both visualisation of the alpha helix using cryo-EM and strong dopamine binding at the active site.
More information on the interactions between TH and dopamine were revealed using molecular dynamics simulations. The team showed that when dopamine is chemically bound to TH by the alpha helix, the function of the enzyme is inhibited. When TH is phosphorylated at a specific location, this releases the alpha helix which was blocking the active site, thereby reactivating the enzyme. These two processes are instrumental in regulating the function of TH in the brain.
This study showcases how Diamond is facilitating access and support for researchers from all over the world to make use of cutting-edge cryo-EM facilities.
Professor Valpuesta says:
Diamond is a reliable place for us to collect good quality data. The instruments at eBIC are very well-maintained, free to access, and our experiments are supported by a professional team of staff. The ability to operate the cryo-EM remotely also means that there are no longer logistical barriers to carrying out experiments.
By revealing the full-length structure of TH and the pivotal role played by the alpha helix in regulating TH activity, this work constitutes a leap forward in understanding the structure-based mechanism underpinning some Parkinsonisms. A better structural understanding may also support the development of novel stabilising/chaperoning therapies to target malfunctioning TH, very much needed by patients who are unresponsive to conventional medications for Parkinson’s.
To find out more about eBIC or discuss potential applications, please contact Principal Electron Microscopist Daniel Clare: [email protected]
Bueno-Carrasco, M.T., Cuéllar, J., Flydal, M.I. et al. Structural mechanism for tyrosine hydroxylase inhibition by dopamine and reactivation by Ser40 phosphorylation. Nat Commun 13, 74 (Jan 2022). https://doi.org/10.1038/s41467-021-27657-y
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.