Find out about upcoming events and webinars, and watch past events.
You can also explore Diamond by using our interactive map and discover the synchrotron, beamlines and much more.
In 2014, Professors Isamu Akasaki, Hiroshi Amano and Shuji Nakamura won the Nobel Prize for Physics for their development of blue light-emitting diodes (LEDs). Although red and green LEDs were developed many years before, producing the blue version was a considerable challenge. The successful development of blue LEDs finally led to the LED-based TVs and computer screens that are now in everyone's pocket, and the bulbs that light our homes. Researchers are currently working on materials for next-generation optoelectronic devices - LEDs and solar cells. Halide perovskites show considerable promise in this field. However, history is repeating itself - although it is now possible to produce efficient and relatively stable red and, especially, green LEDs from perovskites, their blue counterparts are proving more difficult to achieve. In work recently published in Advanced Materials, an international team of researchers demonstrates the use of a polymer to create an efficient and spectrally-stable blue perovskite LED. This novel chemical treatment has great potential for the production of next-generation LEDs across the visible spectrum.
There is considerable interest in developing light-emitting diodes (LEDs) based on halide perovskite semiconductors. The benefits of these materials include excellent colour purity, high luminescence efficiency and tunable light emission. However, although near-infrared, red, and green perovskite LEDs have been developed, achieving good performance with true-blue perovskite LEDs is proving more challenging.
A mixed-halide Cl/Br perovskite system is the easiest way to make LEDs that emit blue light. However, these systems tend to suffer from severe trap-mediated non-radiative losses, limiting their efficiency.
A second issue is that the halide ions in perovskite materials frequently migrate under external stimuli such as light radiation, thermal heating or an electric field. This migration results in the material degrading and its emission spectrum shifting.
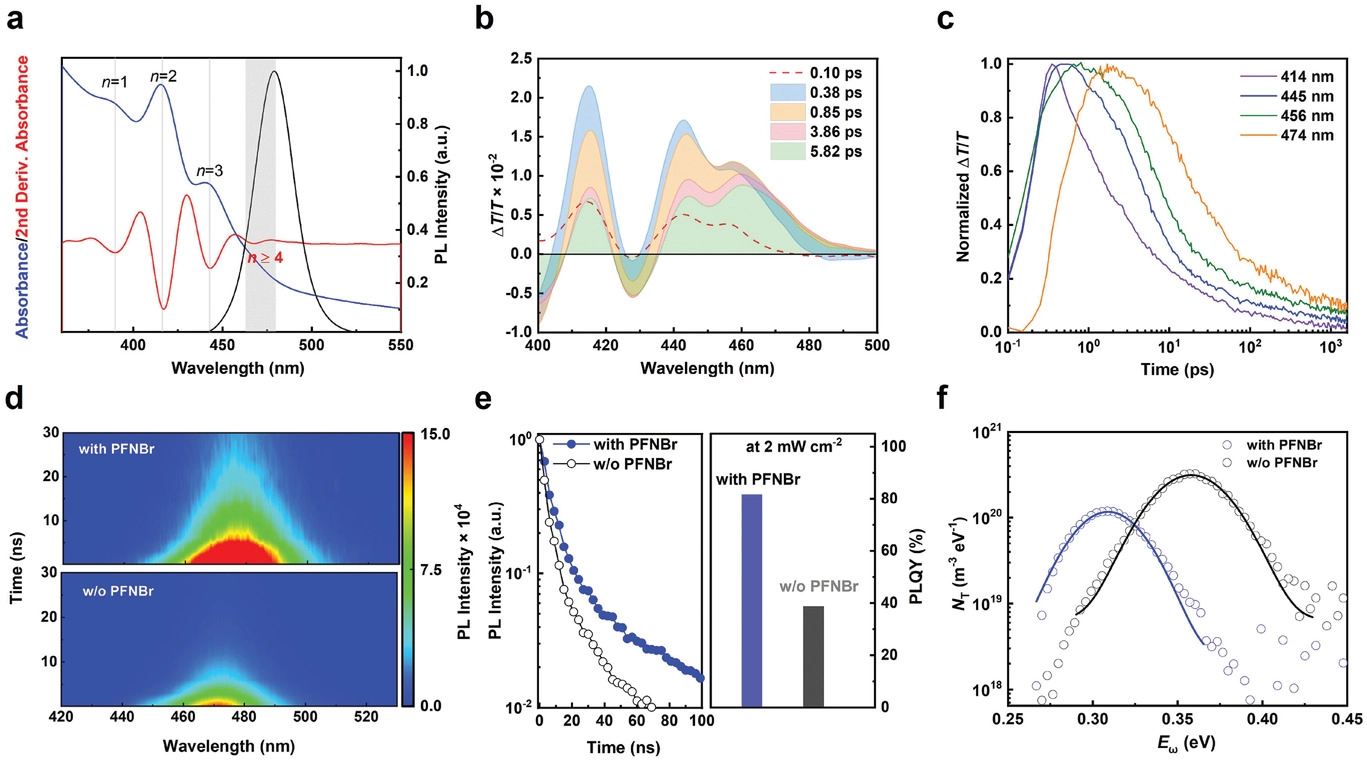
In the work recently published in Advanced Materials, an international team of researchers has demonstrated that adding a fluorene-based π-conjugated cationic polymer (PFNBr) to a mixed-halide Cl/Br perovskite system results in efficient and stable blue LEDs due to a drastic reduction in the trap density.
Mr Edoardo Ruggeri and Dr Miguel Anaya at the University of Cambridge are part of a team specialising in using Grazing Incidence Wide Angle X-ray Scattering (GIWAXS) to investigate optoelectronic materials.
Dr Anaya, explains:
Four years ago, we started investigating perovskite thin films and what happens to them under stress and in different environmental conditions.
That includes light excitation, which can trigger the migration of halide ions. We've been working at I07, the Surface and Interface Diffraction beamline, because GIWAXS allows us to see trace differences in the crystal structure, which we can then correlate with the chemistry and optoelectronic properties of our materials. One of the really useful features at I07 is that we're able to tune how the X-ray beam hits the sample, which means we can see how the material changes in cross-section, even though we're working with thin samples.
Mr Ruggeri, adds:
I07 has a very flexible setup, which allows us to change the sample environment, including the atmospheric conditions. We are also able to integrate our own testing equipment into the beamline. Whereas a lab-based X-ray diffraction exposure can take upwards of 15 minutes, with GIWAXS it's under a second, which means we can see crystal structure changes almost in real-time. We're working towards taking in operando measurements, which will greatly add to our understanding of these materials.
Using GIWAXS, the team was able to assess whether the polymer was incorporated into the crystal structure of the perovskite material. That turns out not to be the case, but this revealing result is interesting in itself - it means that the polymer is playing an electronic role in the compound rather than a structural one. The study demonstrates that PFNBr enhances LED efficiency stability by passivating defects and healing grain boundaries. The polymer also enables better charge transport properties for the devices. Hence this novel chemical treatment offers substantial potential for the development of efficient and stable perovskite-based LEDs covering the entire visible spectrum.
The next steps for this research include investigating more perovskite LED materials and a range of other materials that may replicate, or improve upon, the effect of the polymer. The team are also researching different LED architectures, including different contact materials.
To find out more about the I07 beamline or discuss potential applications, please contact Principal Beamline Scientist Francesco Carlà: [email protected].
Yuan S et al. Efficient and Spectrally Stable Blue Perovskite Light‐Emitting Diodes Employing a Cationic π‐Conjugated Polymer. Advanced Materials (2021): 2103640. DOI:10.1002/adma.202103640.
Ruggeri Edoardo et al. Controlling the Growth Kinetics and Optoelectronic Properties of 2D/3D Lead–Tin Perovskite Heterojunctions. Advanced Materials. 31.51 (2019): 1905247. DOI:10.1002/adma.201905247.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.