3D-Reconstructed Image of the Zinc Dendrites after the Initial Growth in the Cell with a Porous Separator at 30 mA/cm2
Modern life runs on rechargeable batteries, which power all of our mobile devices and are increasingly used to power vehicles and to store energy from renewable sources. We are approaching the limits of lithium-ion battery technology in terms of maximum energy capacity, and new technologies will be needed to develop higher capacity rechargeable batteries for the future. One class of promising candidates is metal-air batteries, in particular zinc-air batteries that have a high theoretical energy density and low estimated production costs. However, zinc-air batteries present certain challenges, in key areas such as cycle life, reversibility and power density. The formation of metal dendrites as the battery charges is a common cause of failure, as dendrites can cause internal short circuits and even thermal runaway. (Thermal runaway is a sequence of exothermic reactions that take place within the battery, leading to overheating and potentially resulting in fire or an explosion. It is also a problem in lithium-ion batteries, and the subject of ongoing research.) In work recently published in Joule, a team of researchers from Imperial College, London, University College London, the University of Manchester and the Research Complex at Harwell carried out in situ experiments investigating how dendritic growth can cause irreversible capacity loss, battery degradation and eventually failure.
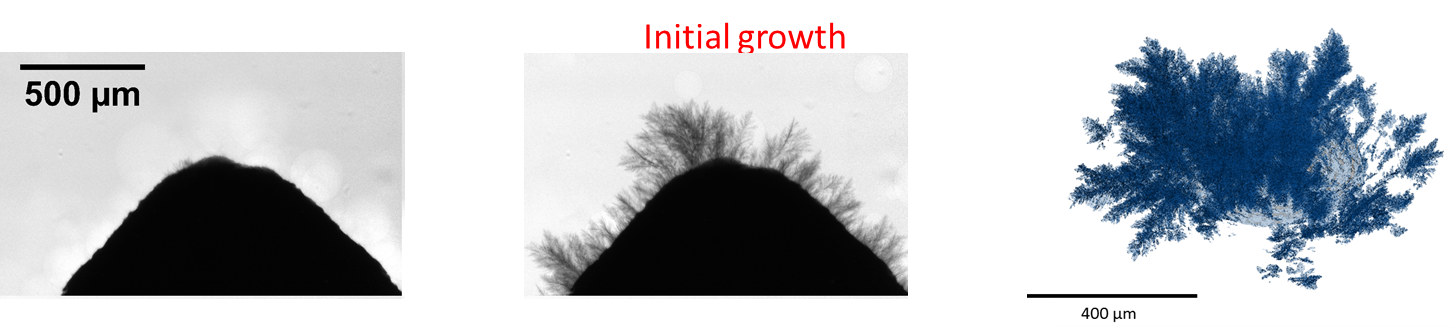
Initial growth of dendrites and 3D visualisation of grown dendrites
Zinc batteries are a promising new battery technology, because they have the potential to offer high energy densities and yet are made from cheap and widely available materials. During the charging cycle, a complex reaction driven by both chemical and electrochemical processes leads to the deposition of dendrites -tree-like elongated crystalline metallic structures - inside the battery. Although the dendrites shrink during the discharging cycle, and their growth can be slowed by the use of certain additives, they do not entirely disappear, and continue to grow during further charging cycles. Dendrite growth can compromise the internal structure of the battery, leading to internal short circuits, and battery failure.

Alleviating the dendrite problem will require a thorough understanding of dendrite formation. Recent advances in scientific instrumentation and electrochemical cell design have enabled some in situ and operando studies of dendrite formation using confocal laser scanning microscopy, nuclear magnetic resonance spectroscopy, transmission electron and transmission X-ray microscopy. X-ray computed tomography has been used to study the behaviour of zinc anodes and air cathodes in zinc-air batteries during operation, but did not capture initial dendrite formation, dissolution and successive regrowth with and without a separator, which is pivotal in providing quantitative evidence for degradation and failure models.
Using synchrotron X-ray computed tomography (SXCT) on the Diamond Manchester Imaging Branchline (I13-2), this study focused on the initial growth, dissolution and regrowth of zinc dendrites in the alkali zincate electrolyte widely employed for static and flow zinc-air and zinc-nickel batteries. SXCT allows non-destructive imaging with high spatial and temporal resolution, and the team used a purpose-designed cell to replicate the behaviour of a large zinc-air cell.

Fig. 3: Initial growth of dendrites through the separator and their 3D visualisation
Their results established that dendrites rapidly start to form on surface inhomogeneities on the electrode surface where the local current is high. Higher operational current densities cause initial dendrite formation to occur more quickly, and the resulting dendrite height to be greater. As the battery is discharged, the dendrites thin and then collapse, detaching from the electrode surface. During the next battery charge, new dendrites form on the electrodes, and previously formed dendrites reattach, forming a complex network. More dendrites form during each charging cycle, with detached dendrites causing temporary short circuits that then become permanent. The presence of a separator significantly affects dendrite growth and morphology, and once dendrites pass through the separator, they do not dissolve entirely and it becomes impossible to prevent short circuits.
The team combined their SXCT results with higher resolution ex situ Focused Ion Beam Scanning Electron Microscopy (FIBSEM) to provide true multi-length scale imaging and accurately capture the precise structure of dendrites down to tens of nanometres. Their findings serve as a basis to begin understanding metal-battery failure mechanisms, and can also benefit other metal-based systems, including lithium or sodium-air batteries.
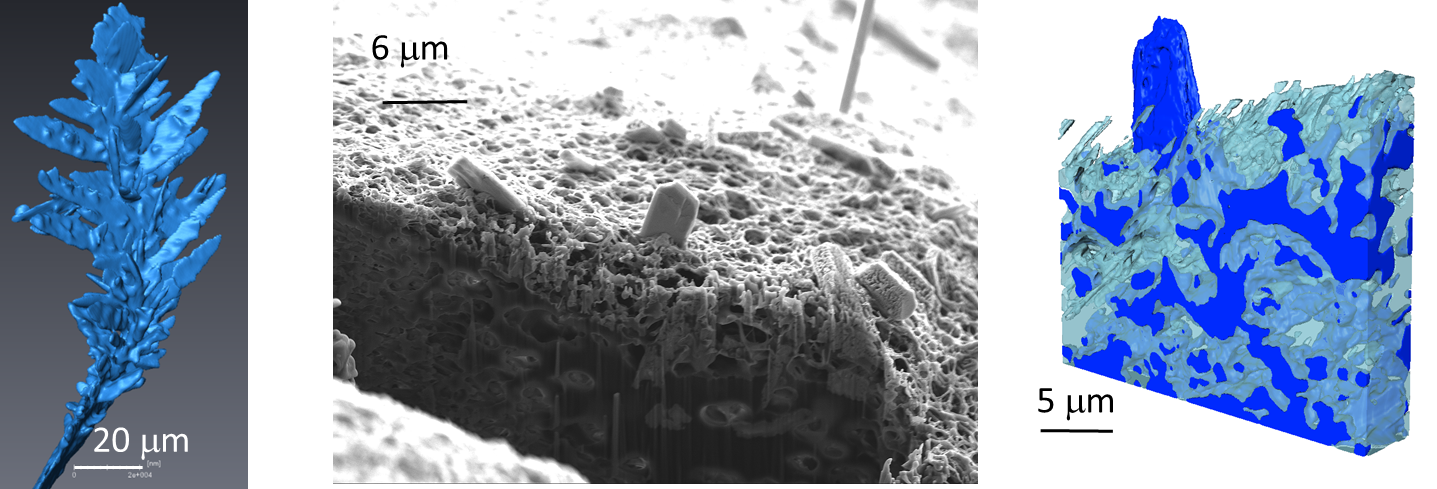
Fig. 4. Single dendrite and dendritic deposits inside and on top of the separator (FIB-SEM)
Lead author Dr Vladimir Yufit, from Imperial College, says:
This methodology provides dynamic and multi-length scale understanding of growth and dissolution mechanisms and the effect of separator on these processes that have never previously been imaged in such detail, which would not have been possible without access to Diamond.
To find out more about the I13-2 beamline, or to discuss potential applications, please contact Principal Beamline Scientist Christoph Rau: christoph.rau@diamond.ac.uk
Yufit, V et al. Operando Visualization and Multi-scale Tomography Studies of Dendrite Formation and Dissolution in Zinc Batteries. Joule (2018). DOI:10.1016/j.joule.2018.11.002.
Radiography of Zinc Dendrite Growth at Current Density of 30 mA/cm2
Radiography of Dissolution and Regrowth of the Zinc Dendrites at 30 mA/cm2
Radiography of Zinc Anode Erosion during 10 Successive Cycles of Dendrite Growth and Dissolution at 30 mA/cm2
Three-Dimensional Reconstructed Image of the Zinc Dendrites after the Initial Growth at 30 mA/cm2
Radiography of Zinc Dendrite Growth at Current Density of 15 mA/cm2
Radiography of Zinc Dendrite Growth at Current Density of 80 mA/cm2
Tomography Image of the Zinc Dendrites after Growth at 80 mA/cm2
Radiography of Zinc Dendrite Growth in the Cell with a Porous Separator at 30 mA/cm2
Radiography of Zinc Dendrite Dissolution in the Cell with a Porous Separator at 30 mA/cm2
Radiography of Zinc Dendrite Regrowth in the Cell with a Porous Separator at 30 mA/cm2
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.