The new European X-ray free electron laser (XFEL) is an international collaboration between 12 countries: Denmark, France, Germany, Hungary, Italy, Poland, Russia, Slovakia, Spain, Sweden, Switzerland, and the United Kingdom. Construction on the project began in 2009, and the facility welcomed its first users in September 2017. Housed in 3.4 km of tunnels underneath Germany, the European XFEL is the first XFEL designed to deliver X-ray pulses with a megahertz inter-pulse spacing, more than four orders of magnitude higher than previously possible. However, it was unclear whether it would be possible to measure high-quality data at megahertz pulse repetition rates because of the damaging effects of the X-ray pulse on the liquid jet delivering fresh sample into the interaction region. Allen Orville, Henry Chapman, and Anton Barty were asked to run community-based experiments for the first beamtime sessions, and they spearheaded ground-state and time-resolved experiments in structural biology. Some of their results, recently published in Nature Communications, show that high-quality structures can be obtained using the 1.1 MHz repetition rate at the European XFEL.
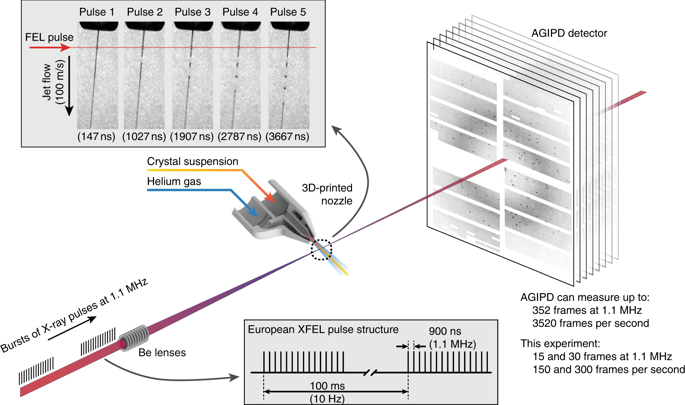
The European XFEL produces bursts of X-ray pulses at a megahertz repetition rate within a train, repeating at 10 Hz frequency. At the current intra-bunch repetition rate of 1.1 MHz the pulse spacing is less than 1 μs, nearly four orders of magnitude shorter than previously available. The decreased time between X-ray pulses delivers more pulses per second while maintaining the same X-ray peak power, but simultaneously poses several challenges for SFX.
The first experiment at the European XFEL was also the first ever to collect SFX data at over 1 MHz, a rate that would be equivalent to 1.1 million pulses if the pulse rate was continuous. Typically, samples are injected into the interaction region as liquid jets; how fast must the jet be to feed this new source? The X-ray beam causes a shockwave to travel up the liquid jet; does this damage the protein crystals on their way to the interaction region before SFX data can be measured? When you’re conducting an experiment with a microcrystal ‘slurry’ and a pulsed X-ray beam just 10-15 microns in size, there are obvious challenges in making sure they coincide in both time and space.
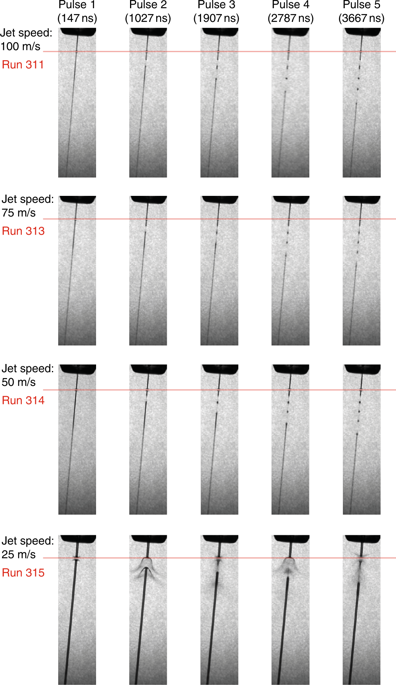
The team showed with direct imaging of the liquid jet using a stroboscopic laser that the XFEL pulse initially vaporises the jet, but the liquid column recovers in time for the next X-ray pulse for jets with a diameter of less than 2 μm and speeds between 50 and 100 m/s, while jets with a speed of 25 m/s do not recover in time.
The researchers were able to produce two complete data sets, one from the well-known model system lysozyme and the other from a previously unknown complex of a β-lactamase from K. pneumoniae. This enzyme belongs to the extended spectrum β-lactamases (ESBLs) that play an important role in emerging multi-antibiotic resistance mechanisms. These result open up megahertz SFX as a tool for reliable structure determination, substrate screening and the efficient measurement of the evolution and dynamics of molecular structures using megahertz repetition rate pulses.
The European XFEL has already performed experiments at 600 pulses per second at 1.1 MHz pulse rates. The instrument has been tested using pulse trains enabling 1760 pulses per second and 3520 pulses per second. It is planned to be available for users in 2019. When the number of pulses in the pulse train is increased to match the maximum AGIPD detector frame rate of 3520 frames per second, the β-lactamase measurements presented here could be completed in under a minute assuming a high crystal hit ratio.
The planned LCLS-II-HE facility promises up to 106 equally-spaced pulses per second, increasing the rate of structure determination even further and enhancing the opportunities for rapid screening for drug targets using on-the-fly substrate mixing. Indeed, rapid data acquisition has the potential for generalising time-resolved movies of macromolecules catalysing their native reaction(s) at physiological temperatures.
However, unlike at a synchrotron, one SFX experiment uses the whole XFEL facility. Consequently, beamtime is both expensive and hard to get (with beamtime granted after rigorous international peer-review). It’s vitally important for users to maximise the data they get from their visit, and yet few users have experience conducting XFEL experiments.
To find out more about the XFEL-Hub, or to discuss potential applications, please contact Principal Scientist Allen Orville: [email protected]
Wiedorn MO et al. Megahertz serial crystallography. Nature Communications 9, 4025 (2018). DOI:10.1038/s41467-018-06156-7.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.