Chromosomes are structures within the nuclei of cells that store DNA. During mitosis, when a cell copies its DNA and subsequently divides, the chromosomes are normally visible using a light microscope, but the resolution of this technique is just 250 nm. More powerful imaging techniques can be employed, but they are limited by requirements for dyes and stains or fail to visualise the chromosome in its entirety.
To see chromosomes in greater detail, a scanning hard X-ray microscope was applied using the longest beamline at Diamond Light Source: the X-ray Imaging and Coherence beamline (I13-1). An X-ray beam was focused down to a nanobeam using a new class of focusing optic known as a multilayer Laue lens (MLL). The hard X-rays plus the MLLs forged a unique imaging tool that enabled a human chromosome to be visualised with nanoscale spatial resolution.
The use of hard X-rays in this scenario was critical for penetrating the entire sample without the need for sectioning, and they allowed absorption-, phase-, and fluorescence-contrast images of platinum-stained human chromosomes to be collected. Scanning hard X-ray microscopy therefore is an extremely valuable imaging tool for examining biological material that could bridge the gap between optical and electron microscopes.
Chromosomes are the central structures of life where the entire genome is organised and stored in the form of a DNA (deoxyribonucleic acid) protein complex. They represent an essential evolution of Eukaryotic life, and determine a cell’s development, reproduction and ultimately death. The Chromosome has a complex hierarchical architecture with feature sizes varying from 2 nm to a few hundred nm, and the structure itself plays an important role in determining the mitotic process of cell division. A variety of microscopy techniques have been employed to image chromosome structures, but each has its own advantages and limitations. Conventional light microscope has a resolution limited to about 250 nm and although powerful fluorescence microscopy methods such as 2D super-resolution imaging by photoactivated localisation microscopy (PALM) have been developed in recent years to extend its resolution to about 50 nm, it is limited to the dyes used. The electron microscope, on the other hand, has sufficient resolution, but electrons can’t penetrate through the micron-sized chromosome. When using electron microscopy to image the internal structure of a chromosome, the use of destructive sectioning processes are usually inevitable.
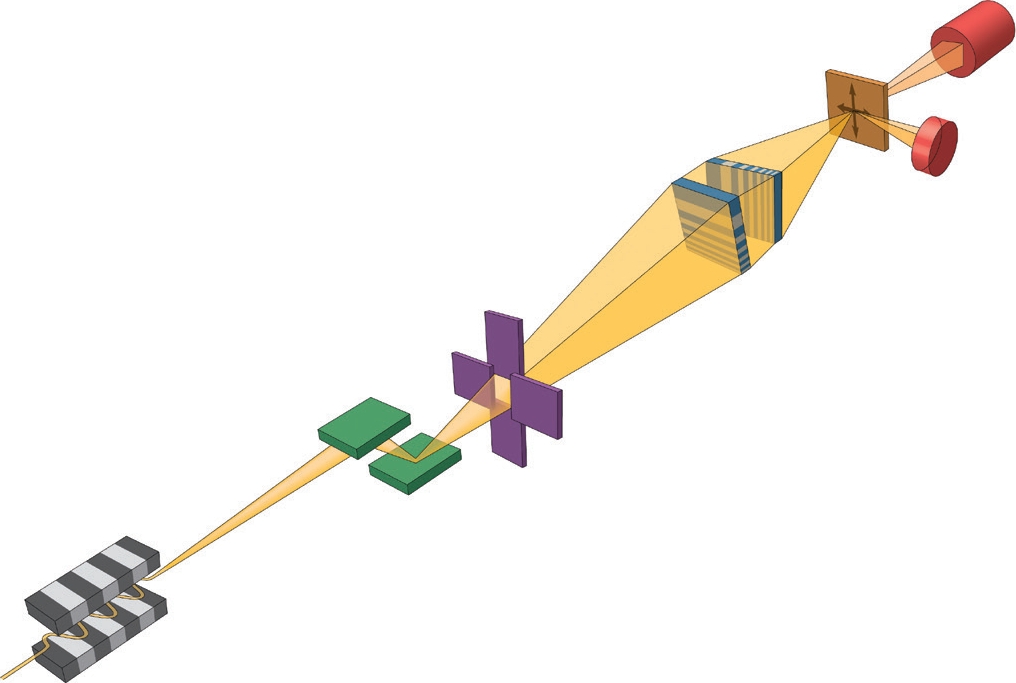
Figure 1: Schematic of the experimental setup. A bright and coherent X-ray beam was generated from the mini-beta undulator source at I13L. A monochromatic beam was focused down to a 13× 33 nm2 spot by two MLLs placed in a cross geometry. In this study because the sample was well isolated, an order-sorting-aperture (OSA) was not needed, which maximised the working distance. A far-field pixel-array detector and an energydispersive detector were used to collect different types of signals as the sample was raster scanned through the focus, thereby yielding absorption-, phase- and fluorescence-contrast images simultaneously.
The X-ray microscope bridges the resolution gap between light and electron microscopes, and bypasses the sectioning problem. Owing to X-ray’s excellent penetration power, it is a non-intrusive imaging tool. Also because of the short wavelength of X-rays, in theory a sub-nm focus can be achieved. However, in practice focusing X-rays down to even nanometer scales is extremely challenging due to the difficulty of fabricating the nanofocusing optics. And it is for that reason that the resolution of X-ray microscopy has hovered around 100 nm and above. In the last decade, the development of novel optics and the advance in nanofabrication process has led to a rapid progress in reducing the focus size, and has pushed X-ray microscopy resolution well below 50 nm.
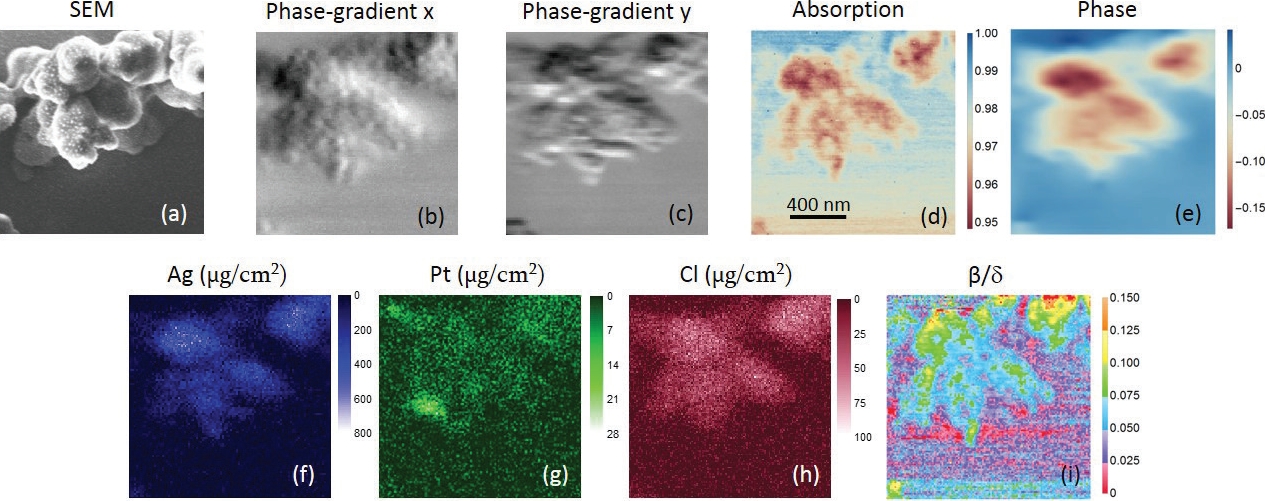
Figure 2: (a) is the SEM image. (b,c) are phase gradient images in horizontal and vertical directions, respectively, in which small globules can be seen. (b) shows more fine features than (c), indicating a better resolution in the horizontal direction. (d) is the absorption-contrast image. Fibrous ultrastructures can be observed. (e) is the phase image (unit in radian). (f–h) are the distributions of Ag, Pt and Cl, respectively. Units are μg/cm2. (i) is the map. Different types of structures can be seen in the centromere and chromatins. The scan-step is 12 nm.
A team from Brookhaven National Laboratory of the Department of Energy, USA, has built a scanning X-ray microscope equipped with a novel optic dubbed multilayer Laue lens (MLL)1,2, which is capable of nanometer focusing. In collaboration with scientists from Diamond Light Source and University College London, they imaged a human chromosome with a resolution of 13 x 33 nm2 at beamline I13-13. Fig. 1 is a schematic drawing of the experimental setup. A pair of MLLs were used to focus the incoming monochromatic beam into a nanospot on the plane where the specimen was located. In a single raster scan, they were able to obtain absorption-, phase- and fluorescence-contrast images all together for quantitative structural and elemental analysis by collecting the fluorescence and the transmitted signals with an energy-dispersive and pixel-array detectors, respectively. Fig. 2 shows the acquired X-ray images of a chromosome. Differential phase contrast was obtained by measuring the lateral shift of the far-field diffraction pattern from the nanobeam. A nonlinear fitting algorithm was adopted to unambiguously determine the phase gradients of the wavefront in two orthogonal directions introduced by the specimen, and the phase was reconstructed by a Fourier integration method afterward. For specimens with weak absorption such as chromosomes, phase contrast is particularly useful for viewing structural variations. The fluorescence-contrast, which measures the type and quantity of the constituent elements of the specimen, gives a quantitative composition map. A combination of all three contrast mechanisms yields additional information that is not available by each alone. The uniqueness of this technique is the simultaneous acquisition of absorption-, phase- and fluorescence-contrast images, which not only eliminates the image registration problem, but also reduces the exposure dose needed without multiple measurements.
X-ray images revealed internal fibrous structures of the chromosome which were invisible with scanning electron microscopy techniques. This demonstrates that scanning X-ray microscopy is a very valuable nonintrusive tool for investigating thick chromosomes, and will open exciting opportunities in biological studies as the result of the hard X-ray’s excellent penetration power and multi-modality imaging and quantitative analysis capabilities.
References:
Funding acknowledgement:
Work at Brookhaven National Laboratory was supported by the Department of Energy, Office of Basic Energy Sciences under contract DE-SC00112704. We acknowledge Diamond Light Source Ltd for providing beam time at I13L.
Corresponding author: Dr Hanfei Yan, Brookhaven National Laboratory, [email protected]
Related publication:
Yan H, Nazaretski E, Lauer K, Huang X, Wagner U, Rau C, Yusuf M, Robinson I, Kalbfleisch S, Li L, Bouet, N, Zhou J, Conley R, Chu Y. Multimodality hard-X-ray imaging of a chromosome with nanoscale spatial resolution. Scientific Reports 6, 20112, doi:10.1038/srep20112 (2016).
Publication keywords:
X-ray scanning microscopy; Phase imaging; Chromosome; Multilayer Laue lens
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.