Thin films of iron oxide, just one layer thick, are interesting both from a fundamental science perspective and for their possible applications. By changing the substrate onto which these films are grown, we can tune their structural and chemical properties, which makes them promising candidates for use in catalysis.
Since the growth of these thin iron oxide films was first investigated in the late 1980s, platinum has been the most studied substrate. In diffraction experiments carried out on the Surface and Interface Diffraction beamline (I07) at Diamond Light Source, researchers investigated iron oxide films grown on silver substrates. Their findings demonstrate that the structure of the film has more of an effect than its other characteristics on how well other materials adhere to its surface - a key step in catalysis. Iron oxide films grown on silver substrates are flatter - less rumpled - than those on other substrates, making it easier for gas molecules to adhere to the surface.
Platinum is commonly used in catalytic converters, helping to control air pollution from vehicle exhausts. However, platinum is a relatively rare and expensive metal, and this research should help us to design new and improved catalysts, that will be cheaper and yet have better performance.
Ultrathin films have recently attracted increased scientific interest due to the uniqueness of their chemical and physical properties. Indeed, the interaction of such a film with the substrate results in significant deviations from the corresponding bulk material from both structural and electronic perspectives. Since ultrathin films may in many cases be considered as two-dimensional materials, their application has a great potential in e.g. heterogeneous catalysis, where the surface of a solid accommodates gas molecules and, thus, plays the key role in the process. Ultrathin iron oxide films in particular exhibit elevated activity in catalytic oxidation and dehydrogenation reactions.
In the one-layer limit, as reported in literature, iron oxide films grown on various square and hexagonal substrates adopt the FeO stoichiometry. Intriguingly, the in-plane atomic structure of such films regardless of the substrate is similar to the (111) crystallographic plane in bulk wüstite, where the (111) termination is electronically unstable due to the polar nature of this surface.
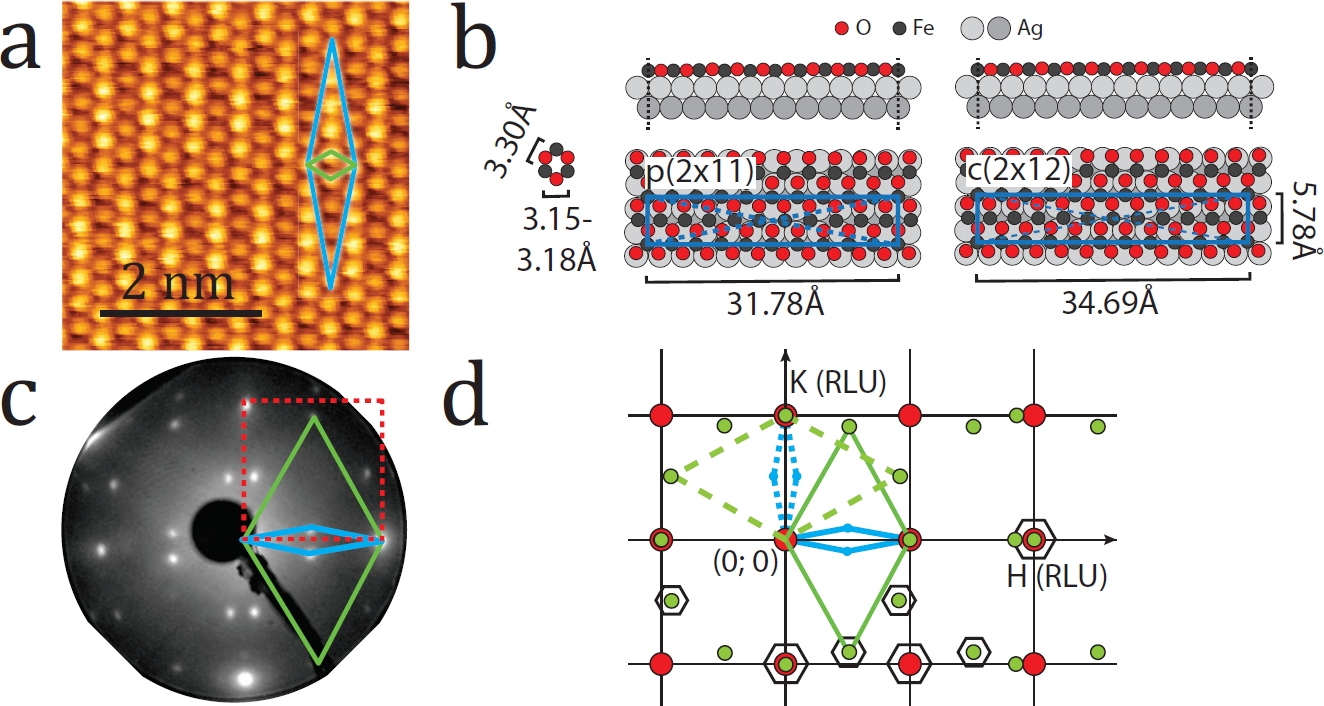
Figure 1: The periodicity and the atomic structure of a one-layer thick FeO film grown on Ag(100); (a) An atomically resolved STM image (-1.0 V, 3 nA); (b) The corresponding ball-model; (c) The corresponding LEED image (40 eV beam energy); (d) The part of the reciprocal in-plane lattice of the FeO(111)/Ag(100) measured with SXRD at the I07 beamline. The substrate, the surface and the coincidence unit cells are marked with red, green and blue colours correspondingly.
The studies of the catalytic activity of complete one-layer thick FeO(111) films grown on Pt(111) in the process of CO oxidation1,2,3 have shown that the catalytic performance of this system under the same conditions is superior than the one of bare Pt(111), which is widely used for CO oxidation in industrial processes. At the same time experiments performed for FeO(111)/Pt(111) exposed to CO+NO at 100 K4 have shown that NO adsorption is negligible. By contrast, it readily proceeds on FeO(111) films grown on a Ag(100) substrate5. This drastic difference and the importance of the interaction of gas molecules with iron oxide films for heterogeneous catalysis have provided motivation for detailed studies of the structure and its influence on the adsorption properties of one-layer thick FeO films. In particular, such systems grown on the substrates that are inert towards CO oxidation deliver an opportunity to exclude the catalytic role of the substrate from the studies.
In the current work ultrathin iron oxide films were grown on a Ag(100) substrate by reactive physical vapor deposition of iron in the presence of oxygen.The Scanning Tunnelling Microscopy (STM) image (Fig. 1a) shows a quasihexagonal atomic arrangement with about 0.4 layer surface coverage (visible on larger scale images not shown here). The lattice parameter of the iron oxide layer is about 3.25 Å corresponding to the (111) crystallographic orientation. A longer-range periodicity observed along the [011] and [0-11] crystallographic directions of the substrate corresponds to a varying coincidence, which on average can be represented as two types of coincidence structure, namely p(2 × 11) and c(2 × 12). The ball models of these atomic structures are shown in Fig. 1b. The corresponding Low Energy Electron Diffraction (LEED) pattern (Fig. 1c) exhibits reflections belonging to the substrate unit cell (red dashed lines), the FeO(111) unit cell and the coincidence structure (green and blue diamonds in both the STM and LEED images).
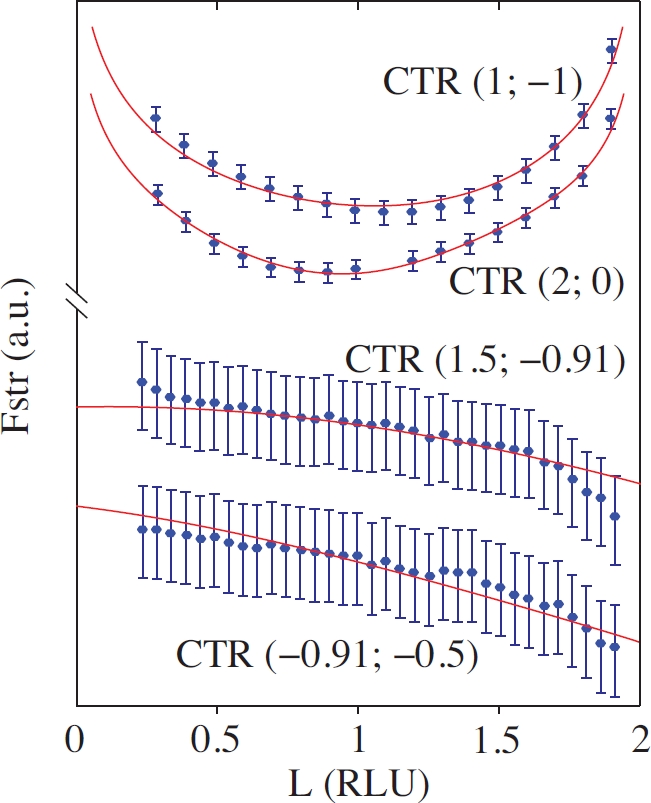
To perform precise structural analysis of FeO(111)/Ag(100), the Surface X-Ray Diffraction (SXRD) setup at the I07 beamline of Diamond light source was employed. The intensity of a number of diffraction rods indicated (Fig. 1d) by black hexagons was measured in the out-of-plane direction providing data for structural fitting. The in-plane positions of the rods at the same time constitute the in-plane map of the FeO(111)/Ag(100) reciprocal lattice corresponding to the LEED pattern. The out-of-plane shapes of some of the measured rods are shown in Fig. 2 together with the best fit achieved based on the structural model proposed by theoretical calculations and refined in accord with the experimentally measured data.
To probe the interaction of the FeO(111) film grown on Ag(100) with gas molecules, NO was used as a common probe of adsorption sites on surfaces. Temperature programmed desorption and reflection absorption infra-red spectroscopy were employed for NO behaviour detection.
The results show that NO adsorbs on top of iron ions with the molecular axis perpendicular to the surface at 100 K and readily reaches the complete coverage of the surface. The results of the described multi-technique study of the FeO(111)/ Ag(100) system supported by theoretical calculations show that due to the weaker interaction of the film with the substrate and better lattice fit, the FeO(111) film on silver is much flatter than the one on Pt(111), where the oxygen atoms protruding outwards sterically block the access of NO molecules to iron ions (Fig. 3). These findings demonstrate that the adsorption properties of monolayer FeO films seem to depend more strongly on the film structure compared with other characteristics of the filmsubstrate interaction. Additionally, it was possible to illustrate the effect of the substrate on structural and adsorption properties of ultrathin films and the potential of SXRD measurements in structural determination of surface structures.
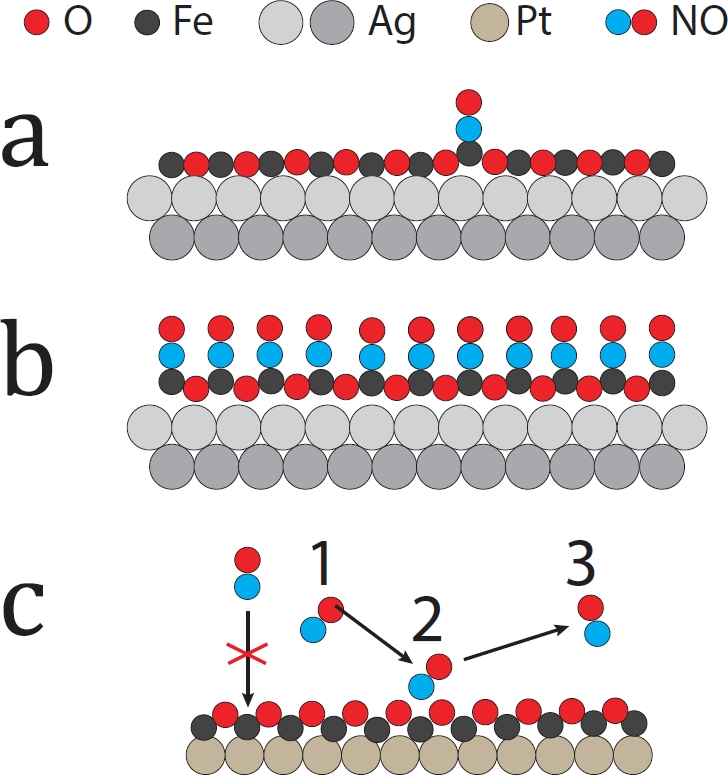
Figure 3: Ball models of the interaction of NO molecules with a one-layer thick FeO film; (a) a low coverage of the film grown on Ag(100); (b) a high coverage of the film grown on Ag(100); steric oxygen blockage of the iron ions of a film grown on Pt(111).
References:
Funding acknowledgement:
The authors would like to acknowledge the Röntgen-Ångström collaboration ‘‘Catalysis on the atomic scale’’. Financial support by the Swedish research council (VR), the Swedish Foundation for International Cooperation in Research and Higher Education (STINT) and the U.S. Department of Energy, Office of Basic Energy Sciences, Catalysis Science Division, Grant DEFG02- 03ER15478 is gratefully acknowledged. The theoretical calculations were performed at C3SE (Göteborg) through a SNIC grant. We would also like to acknowledge Diamond Light Source, UK for experimental time on beamline I07 and thank Adam Warne and Matthew Forster for their help with the experiments.
Corresponding author:
Dr Mikhail Shipilin, Division of Synchrotron Radiation Research at Lund University, mikhail.shipilin (at) sljus.lu.se
Related publication:
Shipilin M, Lundgren E, Gustafson J, Zhang C, Bertram F, Nicklin C, Heard C, Grönbeck H, Zhang F, Choi J, Mehar V, Weaver V, Merte L. Fe Oxides on Ag Surfaces: Structure and Reactivity. Topics in Catalysis, doi:10.1007/s11244-016-0714-8 (2016).
Publication keywords:
FeO; Ag; Surface X-ray diffraction; Reactivity; Single crystal surfaces; Surface structure
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.