G protein coupled receptors (GPCRs) are a large family of proteins that span the cell membrane of eukaryotes and consist of seven transmembrane helices. GPCRs respond to molecules outside the cell and activate signal transduction pathways inside the cell. The adenosine receptor and β-adrenoceptors (βARs) are GPCRs that activate intracellular G proteins in response to the binding of agonists like adenosine or noradrenaline, respectively. The importance of agonist-induced activation of receptors was recently highlighted by structures of βARs that implicate transmembrane region 5 in specific binding to agonists rather than antagonists. This paper presents two crystal structures of a thermostabilized human adenosine A2A receptor (A2AR-GL31) bound to a synthetic agonist NECA and the endogenous agonist adenosine. The structures share all the characteristics of GPCRs that are thought to be in the fully activated state but for the cytoplasmic end of transmembrane helix 6 that partially occludes the G protein binding site. The structures therefore represent an intermediate conformation between inactive and active states. Comparison of the agonist-bound structures of A2AR with those of β-adrenoceptors suggests that contraction of the ligand binding pocket may be a common feature of all GPCR activation.
The simple two-state model of GPCR conformational dynamics describes an inactive state R that preferentially binds inverse agonists and an activated state R* that preferentially binds agonists. R* couples and activates G proteins. Structures of six different GPCRs in conformations resembling the R state have been determined to date and they are highly similar with RMSDs of less than 3Å being observed between any parts of the transmembrane domain. The main structural difference between the R and R* states is a 5-6Å movement of the cytoplasmic ends of helices 5 and 6 away from the receptor core. This reveals a cleft in the centre of the helix bundle where the G protein C terminus can bind. A recently determined structure of an agonist-bound-adrenoceptor (β2AR) in complex with an antibody fragment (nanobody Nb80)1 suggested that the nanobody mimics a G protein by keeping the structure in the activated R* state. Moreover the structure of this agonist bound β2AR is very similar to that of opsin, already considered to be representative of the R* structure of GPCRs. This observation together with the knowledge that the same heterotrimeric G protein can couple multiple different receptors suggests that the R* state of other GPCRs are also very similar. It is however less clear whether the conservation of R and R* receptor structures implies identical activation mechanisms for all GPCRs. Structures of thermostabilized β1AR with four different agonists show that a defining feature of agonist binding is the formation of a hydrogen bond with Ser215 in transmembrane helix 5 together with a contraction of the ligand binding pocket.2 We describe here the structures of adenosine A2A receptor bound to two different agonists that suggest both similarities and differences in the initial action of agonist binding to A2AR compared with βARs. Crystallization and structure solution of the human A2AR bound to the agonists relied on the creation of a four point mutation thermally stabilized construct (A2AR-GL31).3
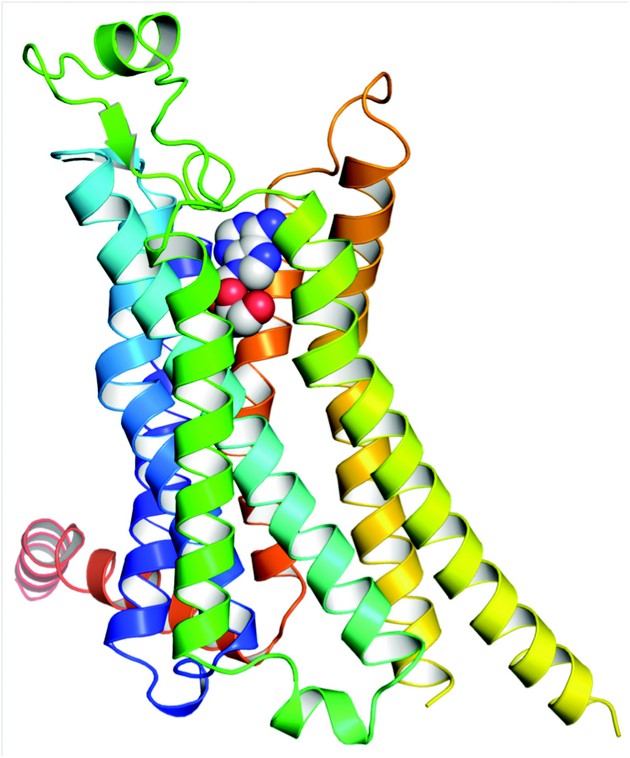
Figure 1: Structure of A2AR bound to its endogenous agonist, adenosine. The receptor is in rainbow colouration (N-terminus blue, C-terminus red) and adenosine is shown as a space-filling model. The extracellular surface of the receptor is at the top of the diagram.
The structures of A2AR-GL31 with adenosine and NECA bound were determined to 3.0 Å and 2.6 Å resolution respectively (Fig. 1). Data for the A2AR-GL31-adenosine complex were measured from four crystals at the I24 microfocus beamline using a 10×10µm2 focused beam. The two structures of A2AR-GL31 were compared with the structure of A2A-T4L, A2AR with T4 lysozyme inserted into inner loop 3 and bound to an inverse agonist ZM241385. Superposition of 96 atoms showing the closest structural homology in the region of the ligand binding pocket (including all residues involved in NECA or adenosine binding) resulted in an rmsd of 0.66 Å. With this alignment the ligands superpose to within 0.56 Å. The largest differences between the two structures are a 2 Å shift of H3 along its helical axis, a bulge in H5 that moves residues by 2 Å into the binding pocket and a conformational change at the cytoplasmic ends of H5, H6 and H7. Comparison with the agonist-bound β2AR-Nb80 structure suggest these differences are similar to those implicated in the formation of the R* state. However, comparison with the structure of opsin bound to the C-terminal peptide of the G protein transducin reveals that there is no room for the G protein C terminus to bind. Assuming that all G proteins act on GPCRs in the same way this leads to the conclusion that A2AR-GL31 does not represent the fully activated state.
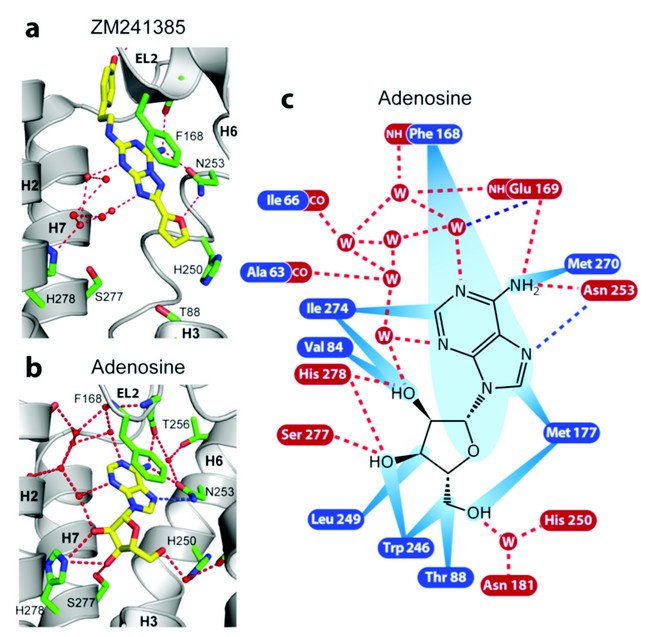
Figure 2: Receptor-ligand interactions for human A2AR bound to a) ZM241385 and b) adenosine. The interactions between the receptor and adenosine (c) are shown as red dashed lines (predicted hydrogen bonds), blue dashed lines (polar interactions) and blue rays (van der Waals interactions).
The key binding differences between the agonist adenosine and the inverse agonist ZM241385 are illustrated in Figure. 2. The furan group of ZM241385 forms a hydrogen bond with Asn253 in H6 and other contacts to residues in H3, H5 and H6. The ribose moiety of NECA and adenosine forms hydrogen bonds with Ser277 and His278 in H7 in addition to other van der Waals interactions with residues in H3 and H6 (see Fig. 2). These clear differences in binding suggest that Ser277 and His278 play a central role in receptor activation, which is consistent with previous studies.4
Agonist binding stabilizes a bulge in H5 that is observed in the agonist-bound structures of both A2AR-GL31 and β2AR-Nb80. The formation of the H5 bulge appears to be a common action of agonists in both βARs and A2AR and inverse agonists appear to block its formation, but the factors contributing to its formation appear to be slightly different in each receptor. In βARs, the direct interaction between the agonist and the side chain of Ser5.46 (superscript refers to the Ballesteros-Weinstein numbering) plays a major role, whereas in A2AR polar interactions with H7 residues along with H3-agonist interactions are mainly responsible. The structure of agonist bound A2AR-GL31 is not fully in the R* conformation and one possibility is that the binding of G proteins is required to stabilize the structure, which has also been suggested fo explain why agonist-bound b2AR in the absence of the nanobody Nb80 remains in an R-like conformation.5 We conclude that the NECA- and adenosine-bound structures are best defined as representing an R/R* intermediate state. The use of Diamond’s I24 microfocus beamline and the ESRF's ID23.2 microfocus beamline was an essential requirement for the determination of these structures.
Agonist-Bound Adenosine A2A Receptor Structures Reveal Common Features of Gpcr Activation, Lebon, G., Warne, T., Edwards, P. C., Bennett, K., Langmead, C. J., Leslie, A. G. W. & Tate, C. G. Nature (2011)
References
- Rasmussen, S. G. et al., Structure of a nanobody-stabilized active state of the β2-adrenoceptor complex. Nature. 469, 175-180 (2011).
- Warne, T. et al., The structural basis for agonist and partial agonist action on a β1-adrenergic receptor. Nature. 469, 241-244 (2011).
- Lebon, G. et al., Thermostabilisation of an agonist-bound conformation of the human adenosine A2A receptor. J. Mol. Biol. (2011) In press.
- Kim, S.K. et al., Modeling the adenosine receptors: comparison of the binding domains of A2A agonists and antagonists. J. Med. Chem. 46, 4847-4859 (2003).
- Rosenbaum, D.M. et al., Structure and Function of an irreversible agonist-β2-adrenoceptor complex. Nature. 469, 236-240 (2011).
Funding acknowledgment
We would like to thank G. Evans, D. Axford and R. Owen for assistance with data collection for the adenosine complex at beamline I24 at Diamond and G. Evans for assistance in preparation of the report. Data on the NECA complex were collected at beamline ID23-2 at ESRF with assistance from D. Flot and A. Popov. The MRC, BBSRC (BB/G003653/1) and Heptares Therapeutics provided financial support.

