Growing concerns about fuel security and global warming have made the development and use of sustainable (low carbon) sources of energy an increasingly attractive proposition. Harvesting energy from sunlight at low-cost using photovoltaic devices is a rapidly growing research area, with organic based photovoltaics (OPVs) attracting particular interest as they have the potential advantages of low manufacturing-cost (as they are fabricated using solution based techniques) and mechanical-flexibility. In an optimized bulk-heterojunction (BHJ), OPVs based on an electron accepting fullerene and an electron-donating conjugated polymer undergo phase-separation into a network-like structure. Following the absorption of light, the excitons created can diffuse to an interface between electron accepting and donating components and undergo dissociation into an electron and a hole. Such separated charges can then be extracted via appropriate charge-conduction percolation pathways. It is known however that to optimize OPV efficiency, it is necessary to control the nanoscale morphology of the donor and acceptor materials that comprise the active semiconductor medium.
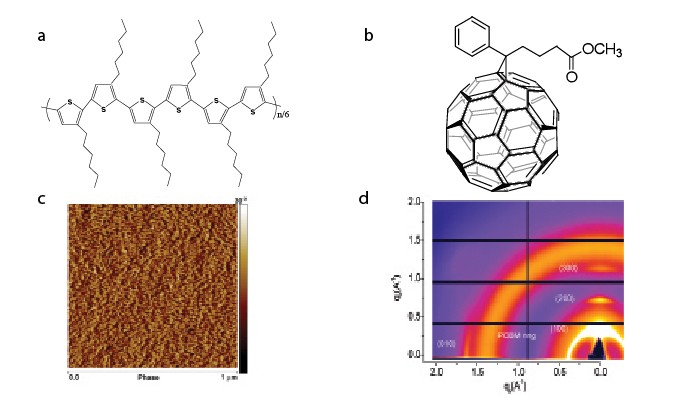
Figure 1: (a and b) The molecular structures of P3HT and PCBM. (c) AFM phase image of a P3HT:PCBM (1:0.8) thin film surface showing P3HT crystal domains. (d) 2D GI-WAXS of a P3HT:PCBM thin film showing the crystal structures of P3HT and PCBM.
Photovoltaic blends based on poly(3-hexylthiophene) (P3HT) and [6,6]-phenyl C61-butyric acid methyl ester (PCBM) (molecular structures shown in Fig. 1) can be used to create devices with a power conversion efficiency (PCE) of 4 to 5%. Previous work to determine the effect of nanoscale morphology on OPV performance has necessarily explored the structures developed in thin-films after they have been prepared. We have however used in-situ grazing incidence (GI) X-ray scattering at beamline I22 at Diamond Light Source to monitor the evolution of P3HT crystallization in P3HT:PCBM photovoltaic blends after solvent casting. This study provides a direct insight into the dynamic self-organization in such complex macromolecular films; an advance that we believe offers the prospect of guiding the formation of thin film-structure and thereby optimizing OPV performance.
After casting from solution, P3HT can organize into ordered crystalline domains (see the AFM phase image in Fig. 1c), PCBM molecules will also aggregate to form nanocrystals. The phase separation between P3HT and PCBM forms a bi-continuous network enabling charge transfer to the electrodes. Figure 1d shows a 2D GI-WAXS image of a P3HT:PCBM blend thin film we recorded at beamline I16 at Diamond Light Source. This 2D diffraction pattern allows us to characterize the crystal structures of P3HT and PCBM phases in the active layer of a solar cell device. The [100] diffraction pattern in the out-of-plane direction indicates a d-spacing of ca. 17 Å corresponding to the P3HT backbones separated by alkyl side-chains, while the [010] diffraction in the in-plane direction indicates a p-p stacking distance of 3.7 Å between P3HT molecular backbones. PCBM aggregates and/or nanocrystals display a broad and diffuse symmetric ring. The crystallization of the P3HT and PCBM phases leads to enhanced optical absorption, charge separation and increased hole mobility. Control of the nanoscale phase separation and crystallization is critical in optimizing the device efficiency.
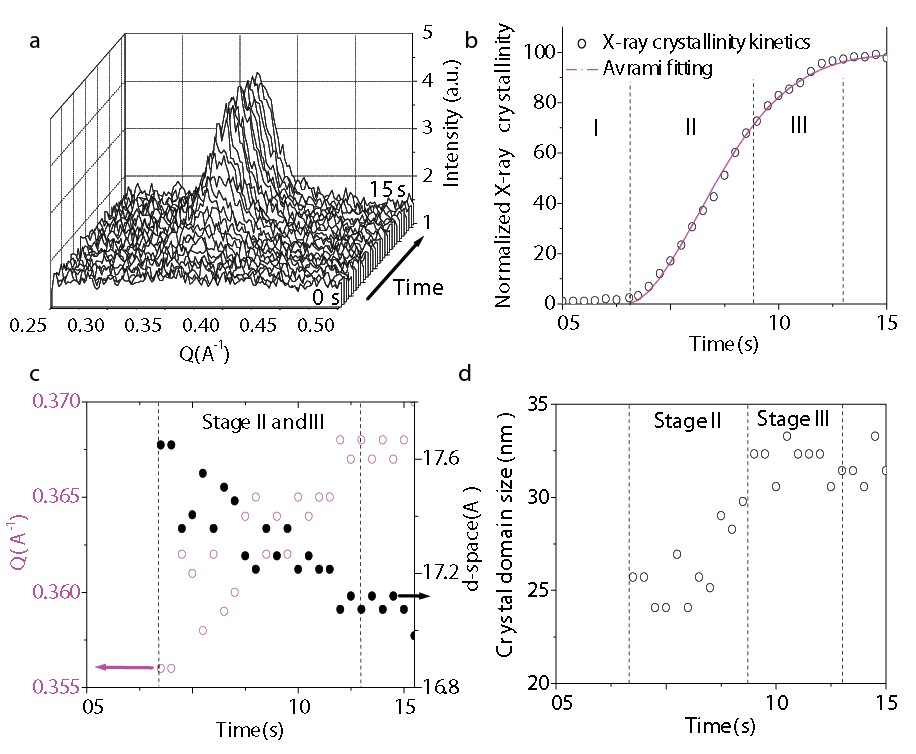
Figure 2: (a) Dynamic GI-XS showing the evolution of the P3HT [100] diffraction peak in a P3HT:PCBM thin film cast from CB onto a substrate at 30oC. (b) The normalized crystallinity determined from the spectra in (a) as a function of time and the Avrami fitting; (c) The time-dependent change in the out-of-plane momentum (Qz) of the [100] scattering and the corresponding d-spacing of the P3HT lamella; (d) The size of P3HT crystal domains calculated from the width of the [100] peak from the Scherrer equation.
Figure 2a shows the time evolution of the P3HT [100] peak from a drying film cast from chlorobenzene (CB) onto a substrate held at 30°C. Figure 2b shows the normalized crystallinity calculated from the [100] scattering peak. It is found that P3HT undergoes rapid crystallization followed by period in which this process slows. An Avrami fitting gives an exponent of n = 1.8±0.1, indicating a heterogeneous nucleation of P3HT from aggregates or impurities. Figure 2c plots the peak-value of the out-of-plane momentum of the scattered X-rays (Qz) determined from the P3HT [100] scattering peak, along with the d-spacing implied by this value. This indicates that as the volume concentration of solvent within the drying fills below 50%, the d-spacing between crystallizing P3HT lamella decreases from 17.7 Å to 17.1Å. We attribute the decrease of the average d-spacing to the effect of self-annealing of crystal defects (i.e. a reduction in kinks, twists, distortions etc). The crystal domain size extracted from the full width at half maximum (FWHM) of the scattering peak shows the average P3HT crystalline domain increases from around (25 ± 1) nm to (32 ± 1) nm as the film fully dries as shown in Figure 2d.
Our understanding of the development of nanoscale morphology during the drying of a P3HT:PCBM blend film is further clarified with the help of spectroscopic ellipsometry performed at the University of Sheffield. The extreme sensitivity of the technique to small variations in film thickness and optical density permits us to follow the evolution of film-structure during its formation process. We found that the P3HT assembles into ordered crystalline lamellae once the solvent volume-fraction in the wet film falls below 50% as a result of solvent evaporation. The rate of P3HT crystallization is initially rapid, but slows when the solvent volume fraction falls below 20%. With the combination of X-ray scattering and spectroscopic ellipsometry, the nanoscale morphology development process can be divided into three stages: (I) rapid solvent-evaporation, (II) slow solvent-evaporation and rapid crystallization, and (III) slow solvent-evaporation and slow crystallization. We found that the degree of P3HT crystallinity in a P3HT:PCBM blend is dependent on a number of parameters including the solvent evaporation rate and the casting temperature. We are using the results to design better film processing conditions to create high efficiency solar cells.
Tao Wang, Alan D. F. Dunbar, Paul A. Staniec, Andrew J. Pearson, Paul E. Hopkinson, J. Emyr MacDonald, Samuele Lilliu, Claire Pizzey, Nicholas J. Terrill, Athene M. Donald, Anthony J. Ryan, Richard A. L. Jones and David G. Lidzey. The development of nanoscale morphology in polymer:fullerene photovoltaic blends during solvent casting. Soft Matter. 6, 4128-4134. (2010)
Funding Acknowledgement
This work is funded by EPSRC via grant “Optimization of polymer photovoltaic devices through control of phase separation” (Grant reference EP/F016433/1, EP/F016255/1, EP/F017057/1 and EP/