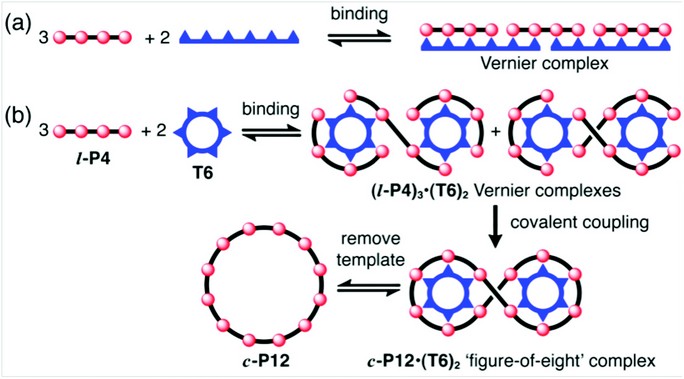
The formation of a Vernier complex between components with different numbers of binding sites provides a way to amplify the molecular length-scale, generating precisely defined assemblies (Fig. 1a). Although the idea of molecular Vernier systems has been discussed for more than 20 years,1 few examples have been investigated experimentally.2,3 We have found that the formation of Vernier complexes between a template and a molecular building block is a powerful strategy for the synthesis of large macrocycles.4 The idea (illustrated in Fig. 1b) is based on the realisation that Vernier complexes do not need to be linear. A template with six binding sites (T6) binds to a building block with four binding sites (l-P4) to form a Vernier complex (l-P4)3•(T6)2, which probably consists of two isomers. The l-P4 units can then be coupled together covalently, to give a figure-of-eight complex, (c-P12)•(T6)2. Displacement of the template gives the free macrocycle c-P12.
We have demonstrated the concept of Vernier templating by synthesising a butadiyne-linked π-conjugated 12-porphyrin nanoring, as shown in Figure 2. Palladium-catalysed oxidative coupling of the linear porphyrin tetramer l-P4 in the presence of the hexa-pyridyl template T6 gave the figure-of-eight complex (c-P12)•(T6)2 in 39% isolated yield. Treatment of this complex with an excess of pyridine, as a competing ligand, resulted in quantitative conversion to the free 12-porphyrin nanoring c-P12 (isolated in 96% yield).
Figure 1: Cartoons showing the concept of Vernier templating. (a) Formation of a molecular 3:2 Vernier complex. (b) Vernier templating: the use of Vernier complex formation to direct the formation of a 12-site macrocycle (c-P12) using a 6-site template (T6).
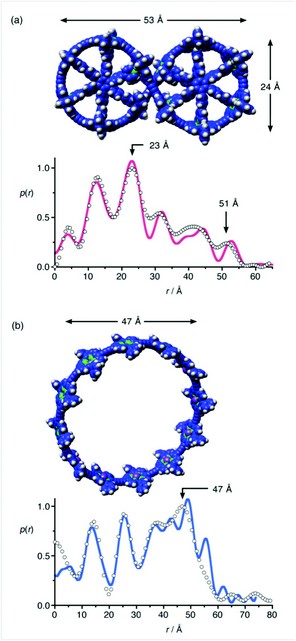
The structures of (c-P12)•(T6)2 and c-P12 were confirmed by solution-phase small angle X-ray scattering (SAXS) data, together with 1H NMR spectroscopy, matrix-assisted laser desorption (MALDI) mass spectrometry and scanning tunnelling microscopy (STM, collaboration with Prof. Peter H. Beton and coworkers, University of Nottingham). Recently SAXS has emerged as a valuable method for characterising synthetic supramolecular architectures.5 The experimental SAXS electron density pair-distribution functions, obtained from dilute solutions of (c-P12)•(T6)2 and c-P12 in toluene (Fig. 3a,b), match well with the simulated pair-distribution functions for calculated geometries (shown). The free nanoring, c-P12, is quite flexible in solution and its SAXS data could only be adequately simulated by using a combination of several elliptical conformations.
The 12-porphyrin nanoring c-P12, with a diameter of 4.7 nm, is among the largest π-conjugated macrocycles ever synthesized. It will be interesting to investigate whether molecules of this type can support persistent ring currents analogous to those observed in mesoscopic metal rings.6,7 Nanorings of chromophores such as c-P12 are also a focus of attention because of their resemblance to natural light-harvesting chlorophyll complexes.8,9 For example the light harvesting antenna complex (LH2) in purple photosynthetic bacteria has a ring of nine chlorophyll units, overlaid with a ring of 18 chlorophylls; its diameter is very similar to that of c-P12.
Our results indicate that Vernier templating will provide access to even larger cyclic chromophore arrays. For example, coupling a five-porphyrin building block, l-P5, in the presence of T6 should give c-P30, by way of a Vernier complex of stoichiometry (l-P5)6•(T6)5. Vernier templating should be applicable to any template-directed cyclisation reaction in which the components have well-defined binding sites, such that a precise mismatch can be set up between nB and nT. This strategy seems to be a general approach to the synthesis of monodisperse macromolecules of a size not previously accessible. The characterisation of these large molecules, which are in the size-range of proteins, has only become possible with the availability of techniques such as synchrotron-based SAXS.
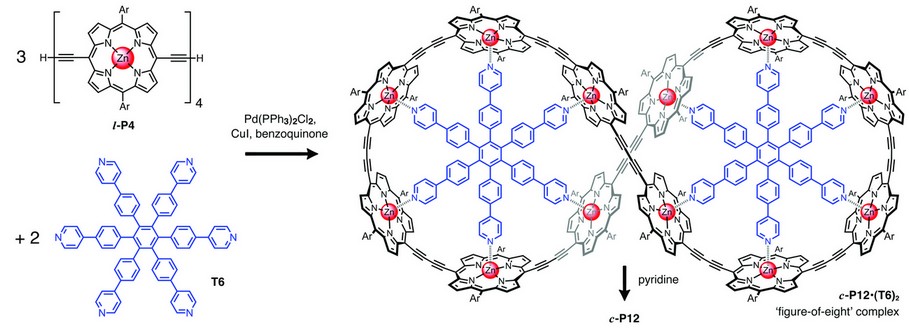
Figure 2: Vernier templated synthesis of the 12-porphyrin nanoring c-P12 via the formation of a figure-of-eight complex c-P12•(T6)2. [Ar = 3,5-bis(tert-butyl)phenyl].
Figure 3: Characterisation of the 12-porphyrin nanoring by small angle X-ray scattering. SAXS pair-distribution data for (a): c-P12•(T6)2 in toluene and (b): c-P12 in toluene/pyridine; experimental points and simulated curves from the calculated structures shown.
O’Sullivan, M.C., Sprafke, J.K., Kondratuk, D.V., Rinfray, C., Claridge, T.D.W., Saywell, A., Blunt, M.O., O’Shea, J.N., Beton, P.H., Malfois, M., & Anderson, H.L. Vernier templating and synthesis of a 12-porphyrin nanoring. Nature 469, 72–75 (2011)
References
- Lindsey, J.S. Self-assembly in synthetic routes to molecular devices. Biological principles and chemical perspectives: a review. New J. Chem. 15, 153–180 (1991).
- Kelly, T.R. et al. A molecular Vernier. Tetrahedron Lett. 39, 3675–3678 (1998).
- Hunter, C.A. and Tomas, S. Accurate length control of supramolecular oligomerization: Vernier assemblies. J. Am. Chem. Soc. 128, 8975–8979 (2006).
- M. C. O’Sullivan, M.C. et al. Vernier templating and synthesis of a 12-porphyrin nanoring. Nature 469, 72–75 (2011).
- Kelly, R.F. et al. Intramolecular energy transfer within butadiyne-linked chlorophyll and porphyrin dimer-faced, self-assembled prisms. J. Am. Chem. Soc. 130, 4277–4284 (2008).
- Mayor, M. and Didschies, C. A giant conjugated molecular ring. Angew. Chem. Int. Ed. 42, 3176–3179 (2003).
- Bleszynski-Jayich, A.C. et al. Persistent currents in normal metal rings. Science 326, 272–275 (2009).
- McDermott, G. et al. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature 374, 517–521 (1995).
- Roszak, A.W. et al. Crystal structure of the RC-LH1 core complex from Rhodopseudomonas palustris. Science 302, 1969–1972 (2003).
Funding
We thank the EPSRC, Diamond Light Source, the European Commission (EU-contract: MRTN-CT-2006-036040, THREADMILL) and the Clarendon Fund for support; the EPSRC mass spectrometry service (Swansea) for mass spectra.