2011 is the International Year of Chemistry and also sees the launch of Sustainability as a University-wide societal engagement theme at Newcastle, so it is appropriate for significant progress to be made in structural characterisation at Diamond Light Source as part of a Newcastle School of Chemistry project developing carbon capture technology and its application in making ‘green’ solvents that, in turn, can be used for environmentally friendly processes to synthesise useful chemicals. The research group of Michael North, pursuing various aspects of organocatalysis, collaborates with crystallographers led by Bill Clegg, making use of both in-house and synchrotron X-ray sources, to promote synthetic methods that are efficient, targeted, environmental and selective, avoiding traditional alternatives that are more harmful and costly.
The catalysis work at Newcastle is part of the broad-ranging interests of the University’s Research Centre for Catalysis and Intensified Processing (http://www.ncl.ac.uk/catalysis/). In recent years complexes of Lewis acidic metals such as Ti, V and Al with salen-type ligands (Fig. 1) have been developed as effective catalysts for various organic reactions, such as the synthesis of cyanohydrins from organic cyanides.1 As a result of small crystal sizes and various structural problems such as disorder and twinning, some of the crystallographic investigations have needed high-intensity synchrotron radiation, and this work began at Daresbury on station 9.8 – a facility constructed in a Newcastle-led project in the 1990s – with studies of some of the vanadium1 and titanium2 catalysts and their derivatives. Synchrotron-based crystallographic investigations in this project subsequently moved to Diamond Light Source when beamline I19 became available in 2008.
Focusing attention on related dinuclear aluminium-based complexes led to the discovery that these could serve as catalysts for the conversion of carbon dioxide CO2 to commercially important cyclic carbonates (see Fig. 1). The reactions can be carried out at ambient pressure and temperature without the use of solvents, and the catalysts are stable for over 60 reuses; there is thus potential for the system to be used to capture waste carbon dioxide from industrial processes and convert it into useful organic compounds (carbon capture and utilisation) rather than the widely proposed ‘carbon capture and storage’ which stores CO2 in a physically unproductive form. Carbon capture and utilisation provides an additional income stream to the operators of CO2-producing facilities (power stations, chemical plants, metal works, cement works, etc.) whilst carbon capture and storage is known to be a highly energy-demanding and hence expensive process to operate. A representative catalytic complex for carbon capture and utilisation was structurally characterised using diffraction data collected in Newcastle.
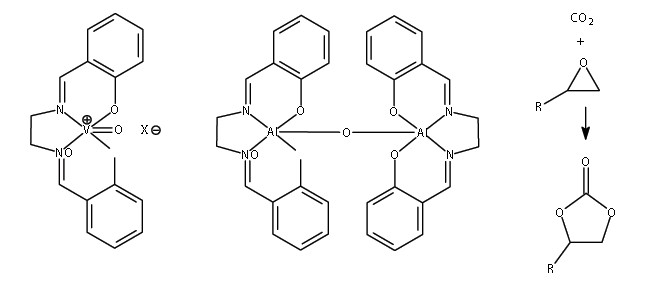
Figure 1: Left: VO(salen)+X- [the salen ligand can carry a range of substituents on the various carbon atoms, not shown here for simplicity]; centre: (salen)AlOAl(salen), the family of catalysts for carbon dioxide conversion; right: the catalysed conversion of CO2 to cyclic carbonates.
What makes this approach even more attractive is that the resultant cyclic carbonates also contribute to sustainable, environment-friendly chemical processes. They can serve as biodegradable and almost odourless solvents of low toxicity, for commercially useful aldol reactions of organic carbonyl compounds, replacing traditional polar aprotic solvents such as dimethyl formamide (DMF), dimethyl sulfoxide (DMSO), environmentally harmful chlorinated solvents, and the carcinogenic hexamethylphosphoramide (HMPA). Such reactions in cyclic carbonates are catalysed by proline, a readily available naturally occurring amino acid. The whole sequence is thus a win-win scenario in both economic and environmental respects. A key aspect of the investigation of cyclic carbonates as solvents for these reactions has been the structural characterisation of some of the products, with a particular need to establish unambiguously the stereochemistry and, to demonstrate enantioselectivity, the absolute configuration of the organic molecules produced. This work has made use of beamline I19 and has been published in the last year.
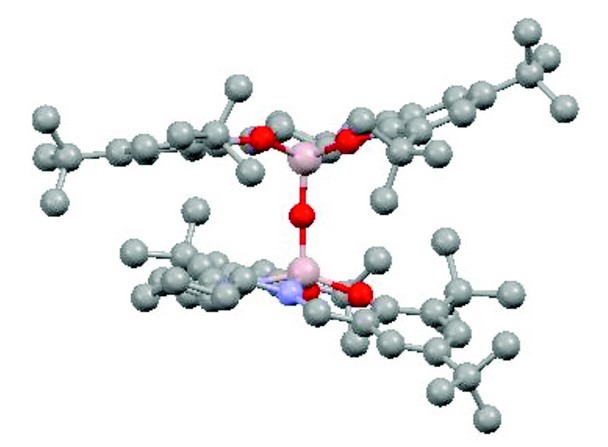
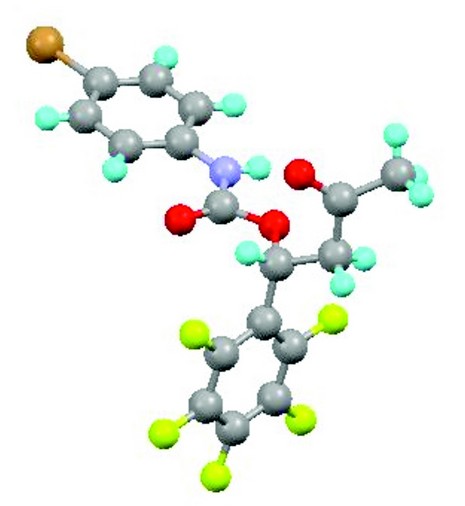
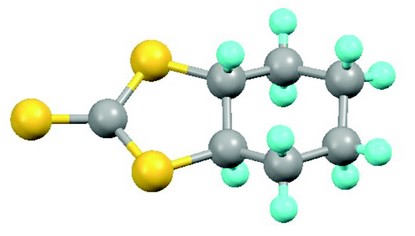
Figure 2: Top: the aluminium-based catalyst for conversion of carbon dioxide to cyclic carbonates (H atoms omitted for clarity; Al pink, O red; N blue; C grey); centre: the absolute configuration of the product of a proline-catalysed aldol reaction (Diamond result; Br brown, F green); bottom: one disorder component of the fully sulfur-substituted analogue of a cyclic carbonate (Diamond result; S yellow).
Having demonstrated the effective and efficient catalytic conversion of carbon dioxide to useful cyclic carbonates, the next stage of the project was to explore analogous reactions with the congener carbon disulfide CS2. The same aluminium catalyst structurally characterised in Newcastle was used to convert CS2 to analogues of cyclic carbonates in which some or all of the oxygen atoms are replaced by sulfur. Here some moderate heating is required, and the actual products formed depend on the temperature. It was particularly important to determine crystal structures in this case, because the existing literature contained ambiguities and, we found through our work, erroneous structural assignments made on the basis of spectroscopic data. Access to Diamond’s synchrotron facilities was crucial, as these structures were rendered problematic by disorder, twinning and a failure to grow large enough crystals for in-house study and we needed to obtain precise and accurate results to provide confident corrections to the literature errors.
The results we have achieved through access to Diamond beamline I19, and to the SRS previously, provide firm structural evidence for the synthetic chemical work. Potential commercialisation of the catalytic processes and useful exploitation of the reaction products are being explored through a recently established spin-out company Dymeryx Ltd, seeking to extend the application to other cyclic carbonates for which there is a major commercial demand, and using power station exhaust gases as the raw source of carbon dioxide.
Clegg, W., Harrington, R. W., North, M. and Villuendas, P. A bimetallic aluminium(salen) complex for the synthesis of 1,3-oxathiolane-2-thiones and 1,3-dithiolane-2-thiones. J. Org. Chem. 75, 6201–6207 (2010).
Clegg, W., Harrington, R. W., North, M. and Pasquale, R. Cyclic carbonate synthesis catalysed by bimetallic aluminium–salen complexes. Chem. Eur. J. 16, 6828–6843 (2010).
Clegg, W., Harrington, R. W., North, M., Pizzato, F. and Villuendas, P. Cyclic carbonates as sustainable solvents for proline-catalysed aldol reactions. Tetrahedron: Asymm. 21, 1262–1271 (2010)
References
- Belokon, Y. N., Clegg, W., Harrington, R. W., Maleev, V. I., North, M., Omedes Pujol, M., Usanov, D. L. and Young, C. Chem. Eur. J. 15, 2148–2165 (2009).
- Belokon, Y. N., Clegg, W., Harrington, R. W., North, M. and Young, C. Inorg. Chem. 47, 3801–3814 (2008).
Funding Acknowledgement
EPSRC, Newcastle University, ORSAS, INTAS, TSB, CarbonConnections



