Crystalline porous solids, such as aluminosilicate zeolites have achieved widespread use in commercial processes of adsorption and catalysis. However, they are limited for many fine chemical conversions of complex organic molecules by the size of their pores, which typically do not exceed 1 nm. Mesoporous silicas are known with pore sizes of 3 nm upwards, but these lack long range order, so that crystallography cannot be used to measure, understand and improve their catalytic performance. Recent developments in the field of metal organic frameworks (MOFs) have shown that the same restrictions on pore dimensions do not exist for these materials, if interpenetration can be avoided. Indeed, several metal carboxylate MOFs such as MIL-1011 can be prepared that have cages with dimensions well within the mesoporous regime (> 2 nm) and openings between these cages between 1 and 2 nm. The same degree of success has not been achieved with metal phosphonate MOFs, however, even though they have been investigated for as long as their carboxylate counterparts. Here we report the structure determination from synchrotron powder diffraction of the novel cobalt phosphonate MOF STA-16 (St Andrews porous material-16),2 the cylindrical channels of which have free diameter approaching the mesoporous regime.
Metal phosphonate MOFs that are fully crystalline and possess permanent porosity are relatively uncommon, but those that are known show good thermal stability due to the strong metal - phosphonate bonding. Recently, we prepared the STA-12 family of metal bisphosphonates, M2(H2O)2L.xH2O (L = N,N’-piperazinebis(methylene phosphonate), M=Mn, Fe, Co, Ni) (Fig. 1).3 These have a pore size of 0.9 nm and are active for Lewis acid and oxidation catalysis. However, the pore size restricts the size of molecule admitted and furthermore adsorption on the catalytically-promising STA-12(Co) is lower than that expected from the crystal structure and observed for the Ni-form, suggesting the presence of defects upon activation. To attempt to circumvent these difficulties, larger pore analogues of STA-12 were targeted. One approach towards the designed preparation of extra-large pore MOFs, termed isoreticular chemistry,4 uses building units of increasing size and with the same coordination geometry to give materials with the same framework topology but increased dimensions, and so larger pores and pore windows.
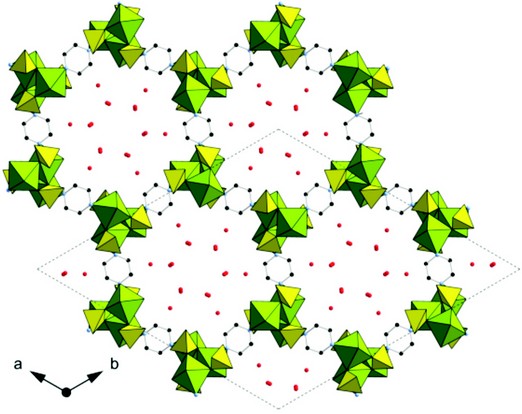
Figure 1: The structure of hydrated STA-12(Ni).
Examination of the topology of STA-12 indicated the structure would be capable of supporting an extended linker possessing a similar geometry to the N,N’-piperazinebis(methylenephosphonic acid) (H4L) used in its preparation. 4,4’-bipiperidine may be thought of as a stretched analogue of piperazine, and thus the modified Mannich reaction was used to prepare the phosphonic acid N,N’-4,4’-bipiperidinebis(methylenephosphonic acid) (H4LL). Crystallographic analysis of H4LL suggested it as a likely candidate for isoreticular synthesis, being centrosymmetric about the bond linking the two rings and so showing similarity to H4L, which is centrosymmetric about the centre of the piperazinyl ring (Scheme 1).
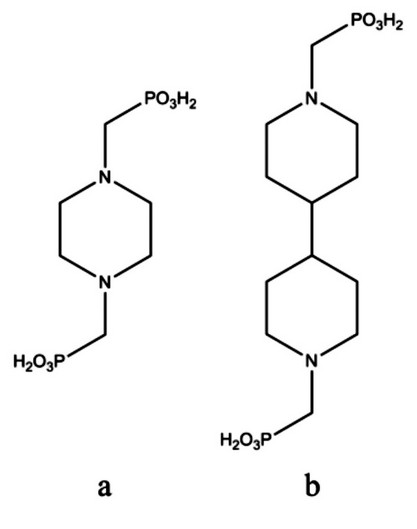
Scheme 1 Bisphosphonic acids (a) H4L and (b) H4LL used in the synthesis of STA-12 and STA-16, respectively.
Initial hydrothermal syntheses performed using cobalt(II) salts and the ligand H4LL gave a range of novel structures, some of which could be established by laboratory single crystal diffraction to be relatively dense materials with little porosity. Among the products of reactions performed at higher dilution was a microcrystalline material which possessed encouragingly high pore volume. However, the presence of cobalt precluded the measurement of high quality laboratory powder diffraction using Cu Ka radiation due to fluorescence, and the obviously large unit cell indicated that its structural elucidation would benefit from the high resolution available at I11 at the Diamond Light Source. The diffraction pattern was therefore measured in a quartz capillary at I11 in Debye-Scherrer geometry using X-rays of wavelength 0.825028 Å. To minimize beam damage, the sample was cooled to 100 K in flowing N2 gas. Eight data sets were collected for three minutes from 1-140° 2?, easily capturing the first, major, low angle peak, with a fresh portion of the sample exposed to the beam by translating the capillary between collections. Data at 2? angles greater than 40° were discarded and the data were re-binned to a step size of 0.003°.
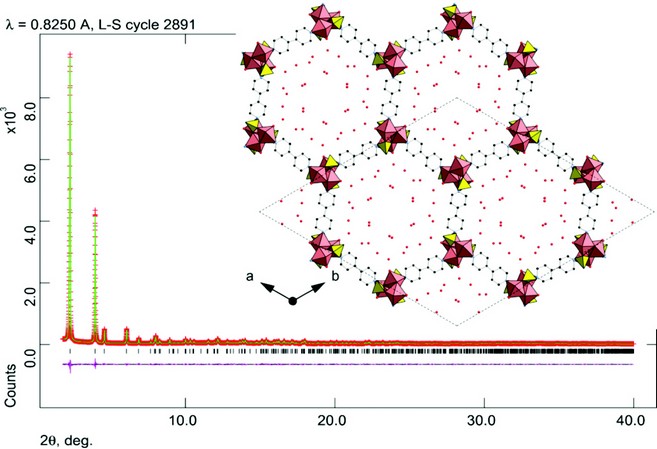
Figure 2: Rietveld fit to the high resolution powder diffraction data from I11, ?=0.825028 Å, with structure of STA-16(Co) overlain. Hydrogen atoms not shown. (R-3, a = b = 41.2870(2) Å, c = 6.2630(1) Å; Rwp = 0.0467, Rp = 0.0338).
The diffraction data were indexed in R3, a = b = 41.2870(2) Å, c = 6.2630(1) Å, a = ß = 90°, ? = 120°. This is the same symmetry as that of STA-12, but with a larger a (and b) dimension consistent with the new material being an isoreticular version of it. To check this possibility, a model was constructed geometrically from the STA-12(Ni) structure3 replacing N,N’-piperazinebis-(methylenephosphonate) (L) groups with N,N’-4,4’-bipiperidinebis(methylenephosphonate) (LL) units. The symmetry of the original solid can be retained, because both ligand molecules can adopt the same local symmetry. The model of STA-16 was also assumed to retain the axial arrangement of the N-coordinating a-aminomethylphosphonate groups which are integral to the structure.
This model was used as a starting point for Rietveld refinement against the diffraction data. Additional positions of physisorbed water molecules were obtained by difference Fourier analysis. The final profile fit is given in Fig. 2 which shows good agreement (Rwp = 0.0467, Rp = 0.0338) between the observed diffraction pattern and that simulated from the refined structure. The final refinement includes 19 independent atoms and 49 restraints and indicates that STA 16(Co) is an isoreticular version of STA 12, in which the short L ligand is substituted by the longer ligand, LL. The final structure of STA-16(Co) is shown inset in Figure 2: viewed down the one dimensional channel system. Spiral chains of edge-sharing CoO5N octahedra are linked by the bisphosphonate ligands, which coordinate at each end through two O atoms and one N atom from the ring. The pore size, measured crystallographically, is 1.8 nm. This is in close agreement with that estimated from the NLDFT analysis of N2 adsorption data. Furthermore, it has also been possible to prepare the nickel analogue of STA-16, which has a similar unit cell to STA-16(Co) and which is similarly porous when dehydrated. Current work is in progress to investigate dehydration behaviour of these materials from diffraction data collected at I11. Dehydration results in removal of physisorbed water and coordinated water bound to the Co and Ni cations, rendering them 5-fold coordinated and therefore Lewis acidic.
The high resolution diffraction facility at I11 is therefore a powerful tool available to the materials chemist to determine structures of complex solids with large unit cells and large pores, in both as-prepared and activated states. As such it will be invaluable in the discovery of novel porous solids of interest in adsorption and separation, drug storage and delivery and catalysis.
Extending the Pore Size of Crystalline Metal Phosphonates toward the Mesoporous Regime by Isoreticular Synthesis, M. T. Wharmby, J. P. S. Mowat, S. P. Thompson, P. A. Wright, Journal of the American Chemical Society (2011) 133, 1266-1269
References
Ferey, G. et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science. 309, 2040-2042 (2005).
Wharmby, M.T. et al. Extending the Pore Size of Crystalline Metal Phosphonates toward the Mesoporous Regime by Isoreticular Synthesis. J. Am. Chem. Soc., 133, 1266-1269 (2011).
Miller, S.R. et al. Structural transformations and adsorption of fuel-related gases of a structurally-responsive nickel phosphonate MOF, Ni-STA-12. J. Am. Chem. Soc. 130, 15967–15981 (2008).
Yaghi, O.M. et al. Reticular synthesis and the design of new materials. Nature. 423, 705-714 (2003).
Thompson, S.P. et al. . Beamline I11 at Diamond: A New instrument for high resolution powder diffraction. Rev. Sci. Instrum. 80, 075107-9 (2009).
Acknowledgements
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.