Spherulites are roughly spherical structures, and their name derives from the Greek for ‘ball’ and ‘stone’. Spherulites occur in various forms in nature. Those that form from polymers self-assemble into arrays of fibres that radiate out from the centre of the structure, and they are typically observed with diameters on the micrometre-scale. The arrangement of the fibres in the spherulite gives rise to birefringence, which may be observed when the spherulite is placed between crossed polarizers on an optical microscope, and many exhibit a characteristic ‘Maltese cross’ pattern of light extinction. Spherulites are on occasion observed in tissue having formed under physiological conditions; they may also be formed in-vitro under non-physiological conditions from a variety of amyloidogenic proteins1. An amyloid protein of particular relevance to Alzheimer’s disease (AD) is the peptide fragment Aß42, a primary component of the insoluble plaques that are present in significantly increased number in specific regions of the diseased brain exhibiting AD. We recently demonstrated that Aß42 can form spherulites under in vitro conditions. Strikingly, this was achieved by the addition of copper, where it was found that copper first dissolved the beta-pleated sheet structure typically formed by self-aggregating Aß42. Having also observed spherulites in Alzheimer’s brain tissue, we were intrigued to determine whether the spherulites formed under physiological conditions bore any resemblance to those being created in-vitro, and whether they were associated with copper in the tissue. We used the microfocus fluorescence mapping facility at Diamond Light Source I18 beamline to investigate further.
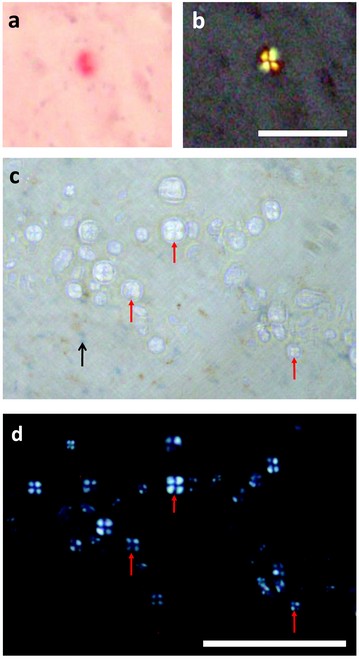
Figure 1: a) Congo-red positive amyloid deposit in Alzheimer’s hippocampus; b) same region viewed with crossed polarisers; c) spherulites from the same tissue that do not stain positive with Congo red; d) same region under crossed polarisers. Scale bar is 100 µm.
Synchrotron X-ray microfocus fluorescence (mXRF) mapping in the hard X-ray region makes it possible to obtain very high sensitivity and specificity data about the distribution of transition metal elements in a material section. This is particularly useful for the examination of biological tissues. Transition metal ions are of interest for their roles in both health and in certain disorders, and they cannot always be detected by conventional staining with sufficient sensitivity (where they are often present in concentrations of ppm or less) or sufficient specificity. Synchrotron mXRF allows both mapping and near-edge-spectroscopy analysis of tissues in order to characterize the distribution and form of transition metal ion deposits.2
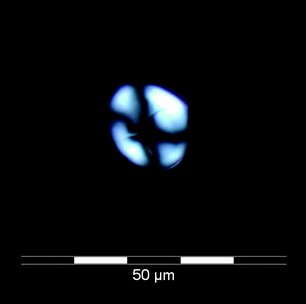
Figure 2: Spherulite formed in-vitro from Aß42 peptide that was incubated in phosphate-free KH buffer at physiological pH and allowed to form ß-pleated sheets (as quantified with thioflavin-T). Copper was added to the preparation, leading to dissolution of the ß-pleated sheet structure and formation of spherulites.
Based on the initial observations that i) spherulites can form in-vitro from the amyloid peptide prevalent in Alzheimer’s disease pathology; ii) spherulites in the in-vitro preparations were only observed subsequent to the addition of copper to the treatment; and iii) spherulites with similar staining properties were observed in hippocampal tissue from an AD case, we performed a mXRF mapping experiment at the I18 beamline to investigate if copper was associated with spherulites in the human brain tissue.
Samples were prepared from the hippocampus region of an Alzheimer’s case (female, aged 90) by cryosectioning to produce 30 µm thick sections that were mounted on quartz slides. The slices included a complete cross-section of the hippocampal horn, and a selection was stained with haemotoxylin (to reveal cell distribution and confirm the position of key boundaries between subfields in the hippocampus) and Congo red (to reveal Alzheimer’s pathology). Amyloid plaques typically stain positive for Congo red, and isolated plaques were observed in regions of the hippocampus. We also observed some staining of the ‘ghost’ tangles associated with the pyramidal neurones. Apple-green birefringence was observed from both the plaques and tangles, but only the plaques exhibited the Maltese cross pattern under crossed polarizers (Fig. 1a, b). In addition to the isolated plaques that stained red and exhibited the apple-green birefringence, we also observed a much higher density of non-Congo-red-positive spherulitic structures, with diameters approximately 5 – 20 mm. These inclusions, which did not show strong affinity for the Congo-red stain or the haematoxylin (Fig.1c) were prolific in the vicinity of the granule layer of the dentate gyrus of the hippocampus, and in a nearby region between the dentate gyrus and the outer Ammon’s horn layer containing the bodies of the pyramidal neurones. The inclusions appeared circular and pale under normal illumination, and had their spherulite properties confirmed with crossed polarizers, with the Maltese cross signature appearing white (Fig. 1d) as opposed to apple-green.
The optical properties and dimensions of these non-staining spherulites in the Alzheimer’s tissue were near-identical to those observed in vitro3 forming from the Aß42 peptide in the presence of copper (Fig. 2).

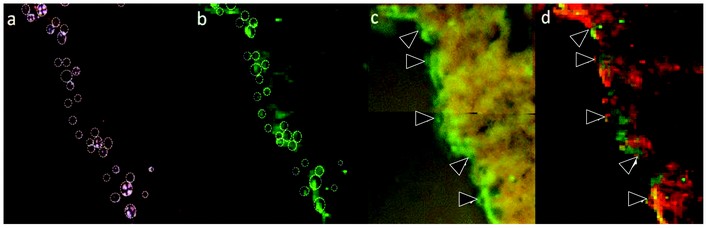
Figure 3: A region of human hippocampal tissue from an Alzheimer’s case, showing a high density of spherulites in a region near a tear in the tissue section. a) Non-Congo-red positive spherulites are shown under crossed polarisers marked with dotted circles. b) The corresponding µXRF map for copper with the spherulite overlay pattern. c) The copper map is computationally overlaid with photographs of the tissue obtained with the beamline microscope camera d) A map of both copper (green) and iron (red) distribution.
Tissue sections were encapsulated on quartz under a layer of Kapton film. Mapping was performed with an incident beam of 10 keV focussed down to approximately 5 mm as incident in the plane of the sample, and the sample stage was stepped in 5 mm increments over the area of interest in order to obtain a map of the region.
Figure 3 illustrates how the spherulite distribution in one of the mapped regions (Fig. 3a) was matched with the copper distribution (Fig. 3b). The copper maps were calculated in PyMCA4 using the area of the Cu Ka peak. The background level of copper in the tissue made it possible to resolve the edge of the tissue in the fluorescence map, and an image of the tissue (captured through the Kapton film on the beamline camera) was overlaid with the copper map using a custom routine in Matlab (Fig. 3c). In order to establish whether the observed variations in copper concentration were predominantly due to uneven sample thickness or edge effects, the map for iron was also generated. Plotting copper (green) and iron (red) together (Fig. 3d) confirmed that the elemental distributions were distinct, and it is evident from Figure 3 that there are locally elevated concentrations of copper associated with the spherulite structures observed in the tissue.
We have not found reports of spherulites with the properties of those illustrated in Figure 3 in the AD literature. It is notable that these spherulites were fragile3, and with diameters of 5 – 20 mm, the spherulites will arguably have been preserved intact in the 30 mm sections to a degree that is not possible in the 5 mm sections typically used for histology. It is possible that spherulites cut open during sectioning are the amyloid-rich deposits that then show a strong affinity for Congo red.
Our findings have raised the interesting question of whether the observed spherulites and Alzheimer’s senile plaques are essentially the same structure, with their formation requiring the involvement of copper.
Exley, C., House, E., Collingwood, J.F., Davidson, M.R., Cannon, D., Donald, A.M. Spherulites of amyloid-ß42 in vitro and in Alzheimer’s disease. J. Alzh. Dis. 20, 1159-1165 (2010)
References
Krebs, M.R.H. et al. Proc. Natl. Acad. Sci. USA 101, 14420-14424 (2004).
Collingwood, J.F.et al. J. Phys. Conf. Ser. 17, 54-60 (2005).
House, E. et al. J. Alzh. Dis. 18, 811-817 (2009).
Solé V.A. et al. Spectrochim. Acta Part B. 62, 63-68 (2007).
Acknowledgements
The human tissue was provided under IRB approval from the Human Brain Tissue Bank at University of Florida, and used with full UK ethical approval. Support was received in part from EPSRC EP/D066654/1, NIH R21NS060304, and the Alzheimer’s Society.
