We show that small quantities of 1,3:2,4-di(4- chlorobenzylidene) sorbitol dispersed in poly(ε- caprolactone) provide a very effective self-assembling nanoscale framework which, with a flow field, yields extremely high levels of polymer crystal orientation. We have used the small-angle X-ray scattering beamline I22 at Diamond to follow in a time resolving manner the formation of the particles and the subsequent crystallisation of the polymer. During modest shear flow of the polymer melt, the additive forms highly extended nano-particles which adopt a preferred alignment with respect to the flow field. These particles have an aspect ratio (length/breadth) which approaches 100. The high aspect ratio means that it is very straightforward to achieve a high common alignment of the nanoparticles using modest flow fields. On cooling, polymer crystallisation is directed by these particles and yields an almost perfect alignment of the polymer crystals. Controlling the morphology and structure in this manner will have a marked impact on the properties of the final material and therefore on their future application in areas such as biomedical devices and scaffolds for tissue engineering.
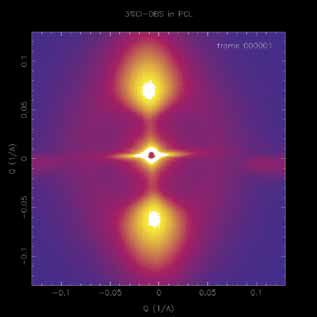 |
| Figure 1: A time-resolved small-angle x-ray scattering pattern of a sample of poly(e-caprolactone) with 3% of 1,3:2,4-di)4-chlorobenzylidene) sorbitol towards the end of the directed crystallisation process. |
Poly (ε-caprolactone) (PCL) is a biodegradable polymer with a swathe of potential applications in biomedical devices including scaffolds for tissue engineering. However, the modest mechanical properties limit progress on these applications. Controlling and directing the semi-crystalline structure of polymers provides one approach for enhancing the properties. Row nucleation provides a powerful mechanism for templating or directing the growth of polymer lamellar crystals. A variety of systems have been identified including macroscopic fibres [1], discrete microscopic particles such as carbon nanotubes [2], and molecular assemblies such as extended chains formed during flow [3]. We have recently shown that dibenzylidene sorbitol (DBS) coupled with modest flow fields can direct the crystallisation of polyethylene [4], polypropylene [5], and poly(ε-caprolactone) [6]. In essence, the low-molar-mass compound self-assembles into extended nano-particles which can be exploited to yield highly anisotropic crystal textures. On cooling, the aligned nanoparticles direct the subsequent crystallisation of the polymer to yield highly aligned crystalline material. This process is dependent on the formation of nanoparticles in the polymer melt as well as the subsequent direction of the nucleation and growth processes of the polymer. These complex processes have been shown to occur in a number of synthetic polymers, and in this work at Diamond we explored the effect of modifying the chemistry of the low-molar-mass compound on the subsequent particle formation and crystallisation processes by considering 1,3:2,4-di(4-chlorobenzylidene) sorbitol (Cl-DBS). The use of a chlorinated sorbitol derivative has the added advantage that the contrast in the X-ray scattering pattern will be greater.
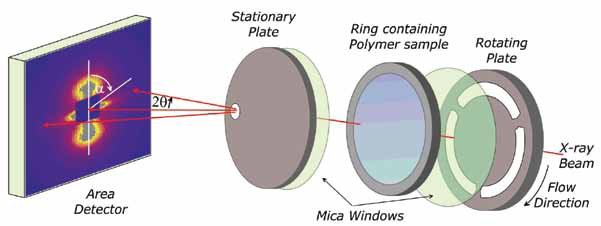 |
| Figure 2: A schematic of the shear cell developed at the University of Reading and used in these experiments on I22. |
We have successfully used the small-angle X-ray scattering beamline (I22) at Diamond to explore the formation of highly extended nano-particles based on Cl-DBS within a polymeric matrix (PCL) under shear flow. We explored how the shear flow affects the formation and alignment of the nanoparticles when cooled from the single phase regime at high temperatures. These nanoparticles act as nucleants for the subsequent crystallization of the polymer matrix and hence we observe two different small-angle scattering features arising from both the nanoparticles and the polymer crystals.
For these studies we mounted a special temperature controlled shear flow cell developed at the University of Reading which enabled controlled and rapid heating and cooling. We were able to obtain high quality time-resolved small-angle scattering data with a time slice of 2s. We were able to establish the temperature that the Cl-DBS dissolved in the PCL upon heating, for a 3% Cl-DBS/PCL system this was ~ 150°C.
| Melt with shear flow | Completed crystallisation state |
 |  |
| Figure 3: A schematic of the directed crystallisation process. | |
These experiments were designed to explore if shear flow can suppress the nucleation and growth of the nanoparticles or indeed enhance the nucleation and growth. The process of nucleation by which embryonic crystals form within a supersaturated solution remains largely unexplained in crystallisation science. In classical nucleation theory, the volume excess free energy of the nuclei at a critical radius balances the surface excess free energy; nuclei larger than this critical radius grow, smaller entities dissolve back in to the solution. We have very tentatively proposed that the shear suppression arises from a shear flow induced reduction in the collision cross-section of the embryonic nuclei thereby limiting the growth of the nuclei to below the critical size. The experiments on I22 revealed that the conditions for observing this behaviour are rather precise. The use of a chlorine derivative of the dibenzylidene sorbitol provided a greater contrast which was particularly helpful in these experiments.
We also studied the nature of the nanoparticles once they had formed. We found that the diameter of the cylindrical particle was ~ 15 nm. The enhanced quality of the scattering data obtained at Diamond showed that the length of the particles was much greater than previously thought. The enhanced quality of the data compared to some previous experiments performed elsewhere allowed more details to be obtained on both the formation and growth of the nanoparticles (greater time resolution) and on their size distribution (extended Q range at low Q). The great resolution of the beamline allowed us to observe that the scattering from the nanoparticles in the form of a horizontal streak was actually made up of a number of discrete components. Analysis of isolated scattering components showed that length of the nanoparticles is ~ 1000 nm. This gives an aspect ratio for the nanoparticles approaching 100. This explains why it is so easy to align the particles using very low shear rates and shear strains. Moreover, it explains why the alignment of the polymer crystals which form upon further cooling is also so high. With a row nuclei extending over 1000 nm there will be large domains of polymer crystal lamellae which exhibit a common or single alignment. The very large aspect ratio of the particle means the level of alignment of the nanoparticles in the shear flow field is also very high. These two factors contribute to an almost perfect alignment of the polymer crystals.
References
[1] D.C.Bassett, Polymer, 47, 5221-5227 (2006).
[2] S. Zhang, M.L. Minus, L. Zhu, et al., Polymer, 49, 1356- 1364 (2008).
[3] J.K. Keum, C. Burger, F. Zuo, and B.S. Hsiao, Polymer, 48, 4511-4519. (2007)
[4] A. Nogales, R.H. Olley,and G.R. Mitchell, Macromol. Rapid Comm., 24, 496-502 (2003).
[5] A. Nogales, G.R. Mitchell, A.S. Vaughan, Macromolecules, 36, 4898, (2003).
[6] J. Siripitayananon, S.Wangsoub, R.H.Olley, and G.R.Mitchell, Macromolecular Rapid Comm., 25, 1365- 1370 (2004).
Principal Publications and Authors
G.R.Mitchell, F.J.Davis, R.H.Olley, and S.Wangsoub to be submitted to the Journal Soft Matter.
Funding Acknowledgement
The European Community’s Seventh Framework Programme [FP7/2007-2013] under grant agreement n° 218331 NaPolyNet and the Science Faculty, Naresuan University, Thailand.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.