A combined time-resolved synchrotron-based Wide-Angle X-ray Scattering (WAXS) utilising a controlled chemical synthesis apparatus including electrochemical monitoring of the reaction was successfully developed to study the formation and transformation of the redox-reactive ‘green rust’ phases. Iron corrosion in some marine environments and waterlogged soils is known to involve the formation of dark olive-green compounds called green rusts (GR) which transform quickly into the usual brown iron rust (i.e. goethite and magnetite) upon further oxidation [1].
The green rust sulphate (GRSO4) formation reaction proceeded via a three stage precipitation and transformation reaction. During the first stage of the reaction schwertmannite (ferric oxyhydroxysulphate; Fe8O8(OH)4.5(SO4)1.75) precipitated directly from solution at pH 2.8 - 4.5. With increasing pH (> 5) Fe2+ ions adsorb to the surface of schwertmannite and catalyze its transformation into goethite (α-FeOOH) during the second stage of the reaction. Ex situ X-ray powder diffraction (XRD) and Attenuated Total Reflectance-Fourrier Transform Infrared Spectroscopy (ATR-FTIR) data agreed with the in situ synchrotron-based WAXS data and provided evidence for the formation of schwertmannite at low pH and its transformation into goethite due to the catalytic effect of surface adsorbed Fe2+ ions. In the third stage of the reaction, the hydrolysis of the adsorbed Fe2+ ions on goethite initiates its transformation to GRSO4 at pH > 7. The proposed mechanism for the formation of GRSO4 is via the hydrolysis of surface adsorbed Fe2+ coupled with electron transfer processes at the solid/solution interface, which lead to the transformation of goethite into GRSO4.
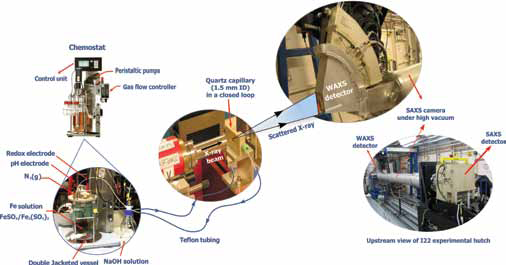 |
| Figure 1: A schematic diagram of the experimental setup on the I22 beamline at Diamond used for the synthesis of GR and the simultaneous collection of WAXS and electrochemical data. The diluted suspension inside the double jacketed reaction vessel was constantly circulated through a quartz capillary mounted in-line with the incident synchrotron X-ray beam. |
The GR family of compounds are mixed Fe2+/Fe3+ solids with a Layered Double Hydroxide (LDH) structure. Each layer in the GR crystal is positively charged and composed of edge-sharing octahedrally coordinated Fe2+ and Fe3+ forming hydroxide sheets, which are intercalated with negatively charged anions (e.g. sulphate) and water molecules. The presence of Fe2+ within the GR structure gives these materials a large capacity to reduce a variety of inorganic and organic environmental contaminants. For example, GR reduces the soluble uranyl ions (UO2 2+) into insoluble UO2(s) nanoparticles [2] and the soluble Cr+6 into the less soluble Cr+3[3]. In addition, breakdown of many toxic chlorinated hydrocarbons such as Trichloroethylene (TCE) can be achieved using GR. These distinctive redox properties make GR a potential candidate for use as a ‘reactive redox barrier’ material for environmental prevention and cleanup technologies such as Permeable Reactive Barrier systems. Nevertheless, the high Fe2+ content in GR means that they transform into ferric phases within minutes of exposure to air which brings an analytical challenge to handling, sampling and characterizing these phases. Conventional ex situ techniques such as XRD and infrared spectroscopy require various sample pre-treatments such as drying, freeze-drying and washing which can bring artefacts when characterising highly reactive materials such as GR. Our recently published paper [4] described a novel synchrotron-based in situ approach for studying and characterising the formation and transformation of highly reactive nano-scale GR compounds.
To accurately characterise GR and understand its formation and transformation pathways we developed a synchrotron-based in situ system to examine the iron–rich nanoparticles as they nucleate, grow and transform in solution. This approach makes use of a chemostat reactor combined with in situ time-resolved X-ray scattering measurements. This modern chemostat allowed precise control, measurement of pH and redox potential (Eh) and maintained strict anoxic environment during the synthesis of green rust. The GRSO4 synthesis was initiated by the progressive addition of NaOH solution to a dissolved Fe2+ and Fe3+ sulphate solution (see Fig 1). The dilute suspensions were constantly circulated through a closed loop consisting of a quartz capillary connected to the reaction vessel. The quartz capillary was aligned with the synchrotron X-ray beam (λ = 1.0 Å) allowing collection of time-resolved WAXS (5 - 65°) data at selected time interval (2 min), for up to 13 hours. This was done simultaneously with the time resolved monitoring of the chemical and electrochemical data which were recorded via an automated control unit attached to the chemostat system.
Analyzing the WAXS data vs reaction time (see Fig .2) allowed quantification of the complex reaction sequences that are controlled by the changes in solution chemistry (i.e., pH and Eh). In this way, short-lived (minutes to hours) precursors or intermediate phases were correctly identified and consequently the GR formation pathways, as well as the mechanisms for each reaction step were derived. WAXS, offline XRD and ATR-FTIR data showed that the nano-colloidal (~ 5 nm) phase formed at pH 2.8 – 4.5 by the hydrolysis of Fe3+ is schwertmannite. Schwertmannite transformed within ~1 h into nanogoethite (< 50 nm) at pH 5.5 – 6. This transformation reaction in our experiment is very fast compared to that occurring in natural systems which require several months to complete. This fast transformation is due to the surface adsorption of Fe2+ ions onto the schwertmannite nanoparticles which initiates an electron transfer between the Fe3+ in the solid matrix and the surface-bound Fe2+. This Fe2+-catalysed process decreases the stability of the schwertmannite crystals leading to structural rearrangements and finally formation of the more stable goethite phase. With increasing pH the goethite transforms gradually into GRSO4. At pH > 7, the solubility of Fe2+ decreases leading to the incorporation of Fe2+ ions in the oxyhydroxide structure and the gradual formation of mixed Fe2+/Fe3+ hydroxide sheets (primary building units of GR) whose excess positive charge is stabilised by the adsorption of sulphate ions. Mechanism of GR formation was further confirmed by the identification of sulphate ions present as part of schwertmannite crystal structure, adsorbed at surface of goethite or intercalated in the GR layers which was characterised across reaction time using ATR-FTIR. WAXS and chemical data showed that as Fe2+ hydrolysis continues to occur, the formation and growth of the Fe2+/Fe3+ sheets continue until GR crystals are well formed. Electron microscopy data showed that the GRSO4 crystals (500 – 800 nm) continue to grow in the a-b crystallographic direction with increasing pH up to 10.
 |
| Figure 2: Three-dimensional plot of time-resolved WAXS patterns collected in situ during the synthesis of GRSO4. The reaction leading to the formation of GRSO4 follows three main stages (i) formation of schwertmannite (pH 2.8 – 5.0) (ii) transformation of schwertmannite into goethite (pH 5 – 6.8) and (iii) formation and growth of GRSO4 (pH > 6.8). Reprinted with permission from Langmuir 26(9): 6593-6603. Copyright© 2010 American Chemical Society, Licensee number 2433720311881. |
This study demonstrated a new approach for the characterisation of complex formation and transformation reaction sequences leading to the formation of the highly redox-reactive GR compounds. The combined electrochemical, chemical and in situ X-ray diffraction analysis allowed us to study fine details of GRSO4 formation reaction under environmentally relevant conditions.
This study showed that schwertmannite and nanogoethite are the only precursors for the formation of GRSO4. The mechanistic information for GR formation is key to understanding its formation and stability in the natural environment, including its ability to decontaminate polluted soils and natural waters.
References
[1] J.M. Génin, P. Refait, G. Bourrie, et al., Structure and stability of the Fe(II)-Fe(III) Green Rust «Fougerite» mineral and its potential for reducing pollutants in soil solutions. Applied Geochemistry, 16, 559-570 (2001).
[2] E.J. O’Loughlin, S.D. Kelly, R.E. Cook, et al. , Reduction of Uranium(VI) by mixed iron(II)/iron(III) hydroxide (Green Rust): Formation of UO2 nanoparticles. Environmental Science & Technology, 37, 721-727 (2003).
[3] L. Legrand, A. El Figuigui, F. Mercier, and A. Chausse, Reduction of aqueous chromate by Fe(II)/Fe(III) carbonate green rust: Kinetic and mechanistic studies. Environmental Science & Technology, 38, 4587-4595 (2004).
[4] I. A. M. Ahmed, L.G. Benning, G. Kakonyi, et al. “Formation of Green Rust Sulfate: A Combined in Situ Time-Resolved X-ray Scattering and Electrochemical Study. Langmuir, 26(9): 6593- 6603 (2010).
Principal Publications and Authors
I. A. M. Ahmed, L.G. Benning, G. Kakonyi, N.J.Terrill, and S. Shaw Formation of Green Rust Sulfate: A Combined in Situ Time-Resolved X-ray Scattering and Electrochemical Study. Langmuir, 26(9): 6593-6603 (2010).
I. A. M. Ahmed, S. Shaw, and L.G. Benning, Formation of hydroxysulphate and hydroxycarbonate green rusts in the presence of zinc using time-resolved in situ Small and Wide Angle X-ray Scattering. Mineralogical Magazine, 72, 1-4 (2008).
Funding Acknowledgement
Natural Environment Research Council (NERC; grant No. NE/ D014026/1) for funding this research. Research was started at the Daresbury Laboratory on MPW 6.2. and was continued at Diamond on I22.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.