In Brief
Professor Trevor Rayment and Dr Alison Davenport from the University of Birmingham have used the Microfocus Spectroscopy beamline I18 to carry out X-ray studies of corrosion that may help corrosion scientists understand the phenomenon of pit corrosion.
"Corrosion resistant" metals are used in challenging environments and where safety is critical, such as in pipelines, aircraft and nuclear waste storage vessels. But it is precisely these materials that are vulnerable to a form of corrosion known as pitting. Developing an understanding of the mechanisms by which these pits form and propagate may allow engineers to build more accurate models of corrosion to predict the lifetime of components and plan when they need inspecting or replacing.
In detail
Corrosion pits can occur in alloys such as stainless steel, penetrating beneath the surface of the otherwise passive metal. Cavities develop beneath the surface in which a highly concentrated acidic metal chloride solution accumulates, causing the metal to dissolve and producing metal cations. When the rate of production of these cations exceeds the rate at which the cations escape from the metal surface, a salt films forms, allowing the pit to grow. Previous studies have examined the formation of these salt films, but the chemistry and structure of the salt layer has been difficult, as the layers are present only on dynamically dissolving metal surfaces.
This study used artificial pits, created by embedding an alloy wire in epoxy resin and dissolving it back. This creates a one-dimensional corrosion pit containing a highly concentrated acidic metal chloride solution characteristic of real pits that can be directly observed with a micro-focused synchrotron x-ray beam. The study was the first to determine directly the structure of salt films formed on dissolving iron, nickel and stainless steel surfaces, by combining data obtained from Diamond’s Beamline I18 and the SRS at Daresbury. The micro-focusing capabilities of I18 made it possible to obtain the high spatial resolution data within the salt film.
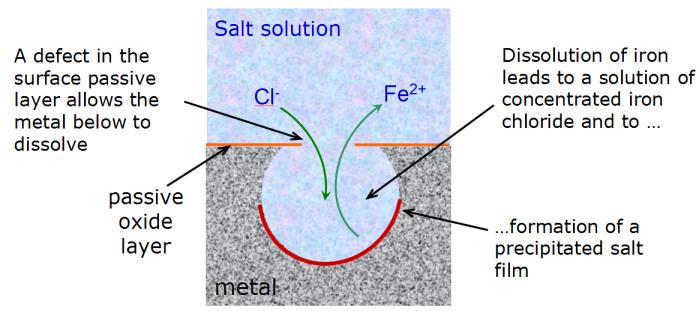 |
| Figure: The mechanism of corrosion pit formation |
The results show that the micro-structure of the salt films formed on iron and stainless steel are quite different. The smooth, continuous diffraction rings observed on iron indicate many fine, randomly oriented crystallites. In comparison, on 316L stainless steel the rings contain a few very intense diffraction spots, indicating fewer, larger crystallites. The size will impact on the transport properties of the salt film, and the roughness of the metal surface.
Prof Rayment says, "This work has also demonstrated that it is possible to observe the mechanisms of pitting corrosion on timescales as short as 10 ms, meaning that transient events can be studied and offering the long term prospect of building structure-based models of pitting corrosion that can predict life-times of affected components."
Trevor Rayment Alison J. Davenport, Andrew J. Dent, Jean-Philippe Tinnes, Richard J.K. Wiltshire, Christopher Martin, Graham Clark, Paul Quinn, J. Fred W. Mosselmans. "Characterisation of salt films on dissolving metal surfaces in artificial corrosion pits via in situ synchrotron X-ray diffraction" Electrochemistry Communications 10 (2008) 855–858
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.