Find out more about our ambitious upgrade project, delivering more brightness, more coherence, and greater speed of analysis to UK science. More about Diamond-II
![]()
Find out more about Diamond's response to virus research.
![]()
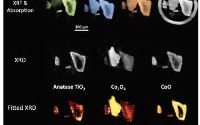
Prof Andy Beale is a long-term Diamond user, including the publication from I18.Read On RCaH at Diamond

A new article just published in Nature Catalysis shows the simple ways of controlling the structure of platinum nanoparticles and tuning their catalytic properties.
Research led by Cardiff Catalysis Institute (CCI) in collaboration with scientists from Lehigh University, Jazan University, Zhejiang University, Glasgow University, University of Bologna, Research Complex at Harwell (RCaH), and University College London have combined their unique skills to develop and understand using advanced characterisation methods (particularly TEM and B18 at Diamond Light Source), how it is possible to use a simple preparation method to control and manipulate the structures of metal nanoparticles. These metal nanoparticles are widely used by industry as innovative catalysts for the production of bulk chemicals like polymers, liquid fuels (e.g., diesel, petrol) and other speciality chemicals (pharmaceutical products).
Meenakshisundaram Sankar of Cardiff Catalysis Institute, who lead this research along with G. J. Hutchings, explains;
Simply by optimising very standard preparation parameters, we show how it is possible to manipulate the structural characteristics of platinum nanoparticles supported on titania to produce a highly active and selective catalyst for the synthesis of functionalised anilines. These products are used in the synthesis of everyday goods such as pharmaceuticals, fertilisers and dyes. This is a huge step forward in understanding the role of preparation parameters in tuning the structure of platinum nanoparticles and this will enable us to design more active metal nanoparticles
The key feature of this article is the tuning the active sites (where the catalytic reaction occurs) in platinum nanoparticles by various heat treatments and using very low platinum quantities to produce an optimised catalyst.
Andy Beale, Professor of Inorganic Chemistry, UCL based at the Research Complex at Harwell, one of Diamond's collaborating scientific facilities adds;
By using ten times less platinum metal, we have substantially reduced the cost of making this catalyst. Even, with lower amounts of metal, the catalyst is either equally active or more active than the current commercial catalyst. Another important advantage is that this catalyst does not produce any undesired by-products (which generates waste and are very expensive to separate and clean up).
Professor Laurent Chapon, Physical Sciences Director at Diamond Light Source concludes;
Catalysis is estimated to be involved in 90% of all chemical processes and in the creation of 60% of the chemical products available on the market, which is why it is studied at the atomic scale. The need to understand catalysis at this level is driven by both economic and environmental concerns; therefore there is a global interest in optimising the synthesis of new catalytic materials and in understanding the fundamental process of catalysis.
At Diamond, we provide specialist analytical techniques for the atomic to microscale characterisation of various catalytic materials, and the in situ study of catalytic processes that are state of the art. We are pleased that the team has been so successful so far in tuning the catalytic properties of platinum nanoparticles.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.