Keep up to date with the latest research and developments from Diamond. Sign up for news on our scientific output, facility updates and plans for the future.
During one of the first user experiments on the Scanning X-ray Microscopy beamline (I08), Dr Jon Hawkings (University of Bristol), Professor Liane G. Benning (University of Leeds and German Research Center for Geosciences), and Professor Rob Raiswell (University of Leeds) peered into the nanoworld of iron chemistry. Their aim was to search for and analyse iron nanoparticles in glacial sediments retrieved from the Greenland Ice Sheet, and icebergs calved off massive glaciers from the high- Arctic archipelago of Svalbard.
Here, Dr Jon Hawkings gives us the lowdown on the importance of iron in our oceans, and how experiments on I08 have helped him and his team investigate how these essential nanoparticles are distributed once they have been released from their glacial hiding places.

Leverett Glacier from the Greenland Ice Sheet. Courtesy of Jon Hawkings.
It is well known that around 30-50% of Earth’s oceans are iron deprived1 and the availability of iron in our oceans impacts the growth of phytoplankton – photosynthesising microscopic marine organisms. The life cycle of marine phytoplankton is very important in that they control many biogeochemical cycles, among which the global carbon cycle is of prime importance. Microscopic organisms phytoplankton are at the bottom of the oceanic food chain and despite accounting for only an equivalent of ~ 2 % of the total terrestrial plant biomass, they are responsible for ~ 50 % of global photosynthesis, and thus carbon fixation1. Indeed, much of the oxygen in the air we breathe originates from this photosynthetic reaction chain that is itself dependent on nutrients like iron.
This an ancient process and the ancestors of these little critters have helped drive changes in the carbon cycle over geological timescales2. However, little is known about how they derive their ‘food’ from areas of the ocean where various elements - and in our case iron - is limited. The most well-known region of iron limitation is the Southern Ocean, which surrounds Antarctica. Glaciated regions have historically been thought of as static environments with little influence on global biogeochemical cycles. However, we3-5 and others6,7 have challenged this idea and showed that glaciers and icebergs are an important primary source of nutrients for marine ecosystems. Nevertheless, the distribution and speciation of the iron delivered to the oceans from glacial or iceberg sediments is still poorly understood and we wanted to investigate the hypothesis that speciation is source-dependent, and changes according to the available delivery pathway.
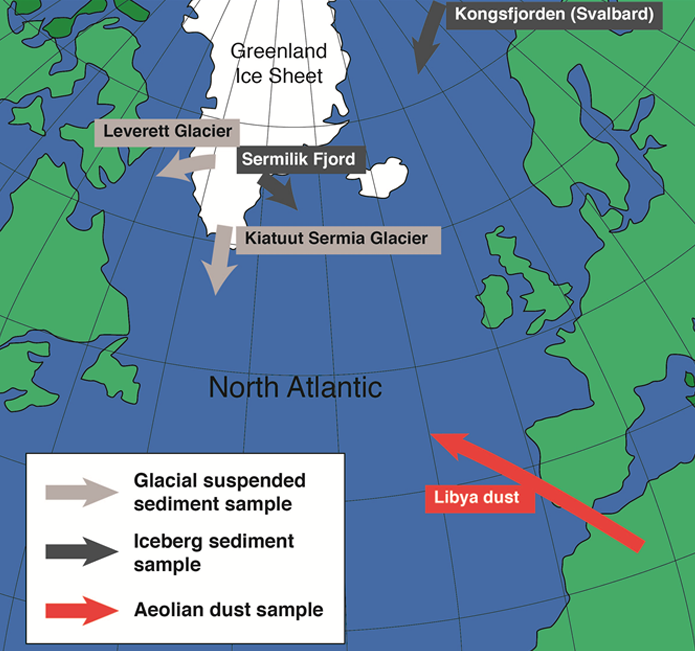
Fig. 1: Sample sites and source type.
Naturally we did our homework, and had already closely studied our samples using traditional chemical extractions and highresolution Transmission Electron Imaging (TEM), as well as nano diffraction and element specific micro-spectroscopic analysis. Yet, we were still missing the capability to map large areas at the highest spatial resolution and evaluate the nanoscale speciation in our diverse and rather complex samples. For that we needed a cutting edge synchrotron approach to give us a novel insight.
Our aim was to be the first research team to investigate the reactivity and bioavailability of nanoparticles from glacial melt waters and iceberg sediments in the Arctic. Basically, we wanted to quantify whether the iron in glacial sediments can be transformed into a form that can easily be utilised by phytoplankton. To do this we mapped out the iron chemistry (Fe L-edge ~700-730 eV) in glacial meltwater suspended particulate matter or iceberg sediment samples from Greenland and Svalbard (Fig. 1), and also analysed a dust sample from Libya (a main Fe-dust source region for the Arctic). We identified regions of interest, analysed these at high spatial and spectral resolution and evaluated the oxidation state and coordination of the nanophase iron minerals (Fig. 2)4,8.
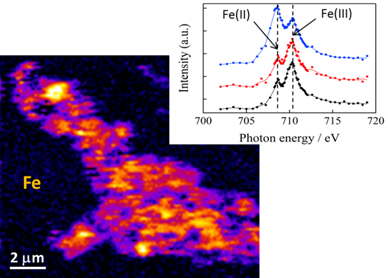
Fig. 2: Example Fe difference map (left) with Fe nanoparticle spectra from three regions of a Greenland Ice Sheet iceberg sediment sample (right). Bright regions of the map indicate higher Fe concentrations. The typical Fe(II) and Fe(III) peaks are marked, and the ratio of these allows identification of nanoparticulate Fe valence state, which gives an indication of Fe lability. Here we see Fe(II) rich (top spectra) and Fe(III) rich (bottom spectra) phase nanoparticles have been identified.
Our measurements were a great success in large part through the dedicated help of the fantastic beamline team. We successfully constructed the first energy-stacked maps and spectral libraries of iron nanoparticle distributions and speciation in Arctic settings. Subsequent analysis of data has identified a wide variety of Fe(II) and Fe(III) nanophases – some of which are highly labile because they contain potentially bioavailable Fe(II) compounds. Data evaluation is in progress but combining this data with our electron microspectroscopic and chemical datasets allows us to assess for the first time how the changing iron chemistry in sediments from glacial run-off or icebergs across the Arctic regulates export of labile iron into the Arctic and northern Atlantic oceans. It goes without saying that our work on I08 would not have been possible without the enthusiasm, dedication and hard work (even at strange late hours) of the I08 beamline team with Burkhard Kaulich, Majid Kazemian Abyaneh and Tohru Araki. Thank you for the support, and we are looking forward to future fruitful collaborations!
To find out more about using the I08 beamline, or to discuss potential applications, please contact Principal Beamline Scientist Dr Burkhard Kaulich: [email protected]

Jon Hawkings
@jonnyhawkingsI’m a post-doctoral research associate at the School of Geographical Sciences, in the University of Bristol. My research focuses on the biogeochemistry of the coldest areas of our planet. Specifically I’m looking at the impact that melting ice sheets may be having on downstream and marine ecosystems. I enjoy working in some of the most inhospitable and challenging environments - pretty much all my data stems from samples collected in the field.

Liane G. Benning and Rob Raiswell discussing the data collected on I08.
Liane (@LianeGBenning) is Professor in Experimental Biogeochemistry at the University of Leeds and Professor of Interface Geochemistry at the German Research Center for Geosciences, GFZ, Potsdam. Rob (@RobRaiswell) is Emeritus Professor at the University of Leeds.
1. Falkowski, P. G., et al. Biogeochemical controls and feedbacks on ocean primary production. Science 281 (5374), 200-206 (1998) DOI: 10.1126/science.281.5374.200.
2. Martin, J. H. Glacial-interglacial CO2 change: The iron hypothesis. Paleoceanography, 5 (1), 1-13 (1990) DOI: 10.1029/ PA005i001p00001.
3. Hawkings, J. R., et al. The effect of warming climate on nutrient and solute export from the Greenland Ice Sheet. Geochem Persp Let. 1, 94-104 (2015) DOI: 10.7185/geochemlet.1510.
4. Hawkings, J. R., et al. Ice sheets as a significant source of highly reactive nanoparticulate iron to the oceans. Nat Comms, 5 3929 (2014) DOI: 10.1038/ncomms4929.
5. Raiswell, R., et al. Bioavailable iron in the Southern Ocean: the significance of the iceberg conveyor belt. Geochem T. 9(7), (2008) DOI: 10.1186/1467-4866-9-7
6. Bhatia, M. P., et al. Greenland meltwater as a significant and potentially bioavailable source of iron to the ocean. Nat Geosci. 6(4), 274-278 (2013) DOI: 10.1038/ngeo1833.
7. Hood, E., et al. Storage and release of organic carbon from glaciers and ice sheets. Nat Geosci. 8(2), 91-96 (2015) DOI: 10.1038/ngeo2331.
8. von der Heyden, B. P., et al. Chemically and Geographically Distinct Solid-Phase Iron Pools in the Southern Ocean. Science 338(6111), 1199-1201 (2012) DOI: 10.1126/science.1227504.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.