Keep up to date with the latest research and developments from Diamond. Sign up for news on our scientific output, facility updates and plans for the future.
The Soft Condensed Matter village has a broad reach in terms of scientific coverage of research applications, as well as a variety of experimental methods offered by the four operational beamlines, although its principal focus is in the Life Sciences.
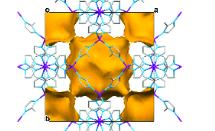 Metal-organic frameworks (MOFs) are a rather new class of micro-engineered materials, possessing flexible frameworks, large surface areas and tuneable pore size. Interest in these materials has grown quickly in the past decade due to their capacity to adsorb gases or solvents, suggesting there is the potential for a wider range of applications than similar microporous structures such as zeolites and molecular sieves. Whilst the current focus for MOFs remains in gas separation and carbon dioxide sequestration for carbon capture, the future looks promising for their usage in fields such microelectronics and biomedicine, amongst others.
Metal-organic frameworks (MOFs) are a rather new class of micro-engineered materials, possessing flexible frameworks, large surface areas and tuneable pore size. Interest in these materials has grown quickly in the past decade due to their capacity to adsorb gases or solvents, suggesting there is the potential for a wider range of applications than similar microporous structures such as zeolites and molecular sieves. Whilst the current focus for MOFs remains in gas separation and carbon dioxide sequestration for carbon capture, the future looks promising for their usage in fields such microelectronics and biomedicine, amongst others.
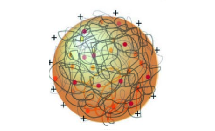 Many scenarios have been proposed for processes leading to the formation of the protocells that are a pre-requisite to life on early Earth. One theory is based on fatty acid vesicles, which form when naturally occurring fatty acids self-assemble in water to form a bilayer membrane. An alternative theory suggests that protocell formation may occur when organic chemicals and naturally occurring polymers present in aqueous solution spontaneously phase separate to form membrane-free chemically rich liquid microdroplets, called coacervates. Combining these two approaches, a hybrid protocell model has been developed based on the spontaneous self-assembly of a fatty acid membrane on coacervate microdroplets.
Many scenarios have been proposed for processes leading to the formation of the protocells that are a pre-requisite to life on early Earth. One theory is based on fatty acid vesicles, which form when naturally occurring fatty acids self-assemble in water to form a bilayer membrane. An alternative theory suggests that protocell formation may occur when organic chemicals and naturally occurring polymers present in aqueous solution spontaneously phase separate to form membrane-free chemically rich liquid microdroplets, called coacervates. Combining these two approaches, a hybrid protocell model has been developed based on the spontaneous self-assembly of a fatty acid membrane on coacervate microdroplets.
Amyloid fibrils are aggregates of proteins that occur when water-soluble proteins are mis-folded such that they are no longer soluble in water. Fibrils can form readily in vitro and their formation has been implicated in many diseases, notably amyloidoses such as Alzheimer’s and Parkinson's disease. Understanding factors that promote or inhibit amyloid development is key to tackling these diseases. Investigation is underway to determine whether all proteins can assemble into amyloid-like fibrils in suitable conditions in the presence of biological initiators.
The Multimode InfraRed Imaging and Microspectroscopy (MIRIAM) beamline (B22) experimental performances are of the highest spectral quality and optical resolution among any broadband IR microanalysis system. In practice, an IR microbeam as small as 3x3 (15x15 at max) μm2 is used for scanning IR microscopy, or full-field IR microscopy via a Focal Plane Array (FPA) detector at high magnification 74x (or 36x), for IR imaging simultaneously over areas of 30x30 (max 70x70) μm2, and effective detector pixel size 0.5 (1) μm.
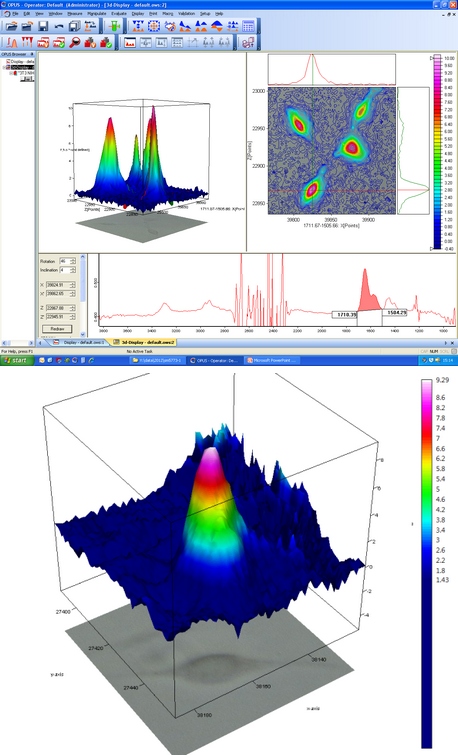 Figure 1: B22 IR imaging by FPA detector of protein concentration in living fibroblast cells and deuterated water. Top: four cells in the 2D, field of view of about 170x170 μm2 by 15x objective and globar. Bottom: single cell in 3D, field of view of circa 70x70 μm2 via 36x objective (effective pixel size ~1 μm) and Synchrotron illumination.
Figure 1: B22 IR imaging by FPA detector of protein concentration in living fibroblast cells and deuterated water. Top: four cells in the 2D, field of view of about 170x170 μm2 by 15x objective and globar. Bottom: single cell in 3D, field of view of circa 70x70 μm2 via 36x objective (effective pixel size ~1 μm) and Synchrotron illumination.
Among the main scientific achievements in the Life Sciences at MIRIAM is the proof of principle that SRIR can image the biochemistry of living cells in real time. Insight on cell life-sustaining chemistry and control of cellular metabolic activity are key targets of medical doctors/biologists and for pharmacological research; for example in cancer cell screening or stem cell manipulation. Results published in collaboration with staff from the Paul Scherrer Institut (PSI) in Switzerland and Diamond1 have shown in vivo the metabolic activity of fibroblasts, the most common type of cell in the connective tissue in animals and humans. Cells were grown for days in Diamond’s Cell Culture Laboratory, and then placed into a sample holder with heavy water (D2O) under the IR microscope. By tracking in time deuterium (D) – initially outside the cell – isotopic pathways in and out of the cell were monitored. IR images clearly showed not only gradients of isotopic concentration (water more or less enriched in H or D) around the cell, but also the D isotope incorporation into the proteins inside the cell. With the study of metabolic processes now possible in real time at single cell level, a wealth of new information on cell biology will become available as scientists learn how to use this approach.
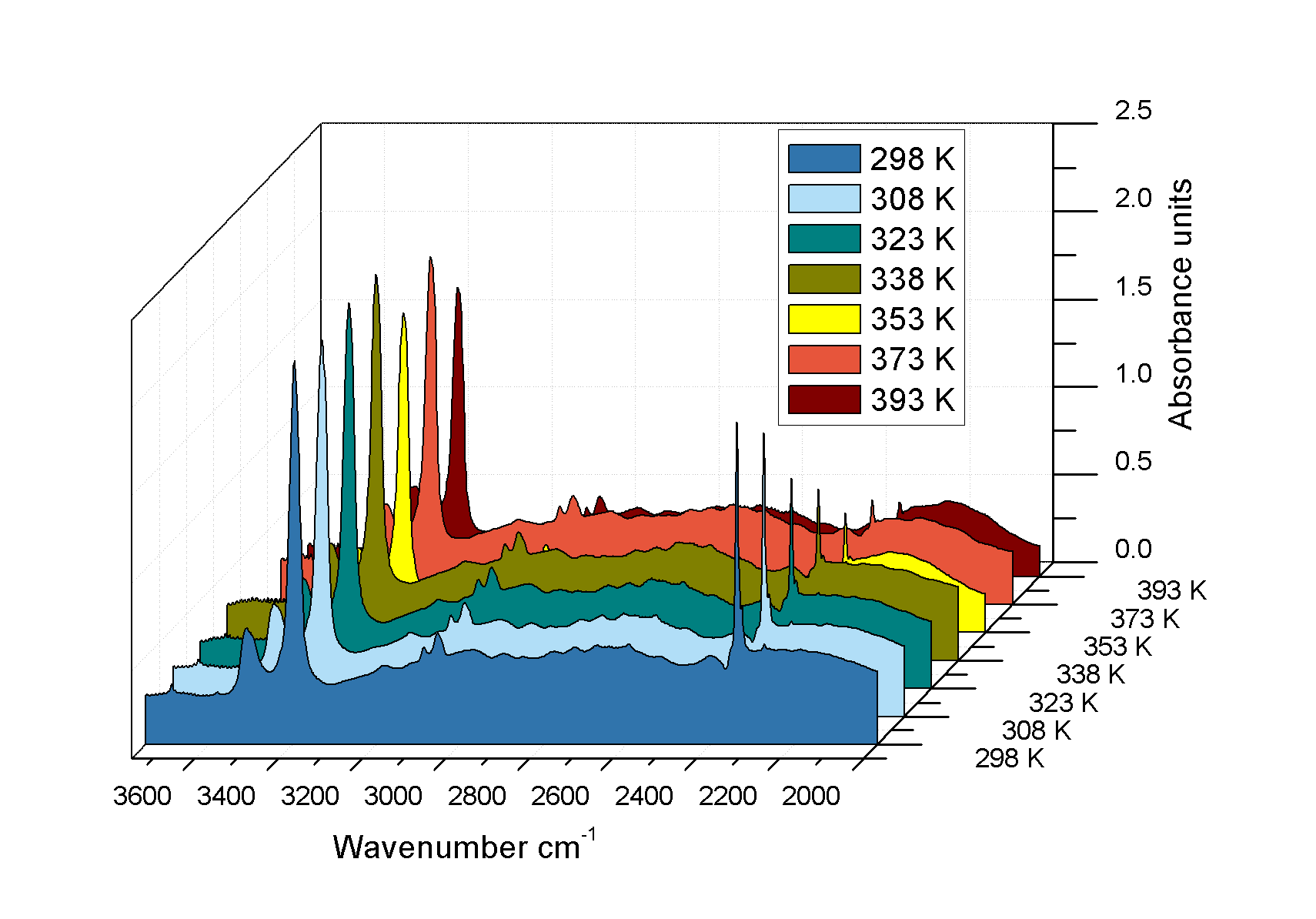
Figure 2: Inset: Image of single crystal of MOF Sc2(NH2-BDC)3 (BDC=1,4-benzenedi-carboxylate) under IR microscope via 36x objective showing 15x15 μm2 areas (green boxes) selected for IR analysis. Main: IR microspectra collected during CO2 uptake at a partial pressure of 0.1 bar over a temperature range 298 – 373 K. As temperature increases the magnitude of the adsorbed CO2 asymmetric stretch modes at 2335 cm-1 decreases relative to the NH2 symmetric and asymmetric ones.
A complete new class of microIR experiments has been pioneered in the last couple of years at MIRIAM in the Physical Sciences, namely novel in situ SRIR microanalysis of gas-solid interactions. Started as a follow up of beamtime from St. Andrews and Aberdeen University researchers, in collaboration with MIRIAM staff at Diamond, it has developed into a powerful new SR FTIR microspectroscopic technique that permits the rapid analysis of adsorption by porous materials under flowing gas conditions. The significant social aspect of this field of applications is in the important use of small pore zeolites/zeotypes as fast catalysts, either in the synthesis of plastic precursors, or in the highly effective removal of nitric oxide (NO) from the emissions of lean burn automotive engines2. Specifically, in situ SRIR absorption spectroscopy on microcrystals (<35 μm) was used to monitor the dehydration of the copper based organic complexes – used as concentrate for micropore into silicoaluminophosphate – at high temperatures (400 °C). Polarised SRIR light also revealed the strong alignment of N−H bonds in the anisotropy crystal.
In the past 12 months, SRIR microspectroscopy has enabled the determination of carbon capture in a new engineered material; metal-organic frameworks (MOFs). There is a current focus on carbon sequestration/capture from flue gases due to global warming and the greenhouse effect from CO2 accumulation in the troposphere. The brilliant and polarised SRIR source at MIRIAM was used for pinpointing absorbance changes of the stretching modes of adsorbed CO2 molecules aligned along the channels of the MOF pores, even with uptakes as low as <0.1 mmol/g (fractional site occupancies ~1/70), a result experimentally difficult to attain by other means. In perspective, the method has the potential to follow temporal and spatial variation over a single crystal and to determine adsorbate diffusivities.

A unique characteristic of the Circular Dichroism (CD) beamline (B23) is the highly collimated microbeam adjustable from 300x500 μm2 to 1x2 mm2, which allows SRCD on an extremely small volume of sample using narrow cuvette (few mm pathlength) or long capillary (up to 10 cm). This is key to enable successful measurement of highly diluted biologically important proteins (few microg per ml) as well as adsorbed onto gold and/or silver nanoparticles. Recently, the B23 microbeam was further exploited in the development of High Throughput Circular Dichroism (HT-CD). The successful tests on 96- and 384-well multiplates mean that HT-CD will be available for the next round of users’ applications (Fig. 3). Complementing the increased range of applications at B23, the current CDApps software (Fig. 4) – developed for SRCD data acquisition, processing and analysis at Diamond – will be tailored for HT-CD, in particular for screening of protein- and/or nucleic acid-ligand binding interactions and optimisation of protein crystallisation conditions.
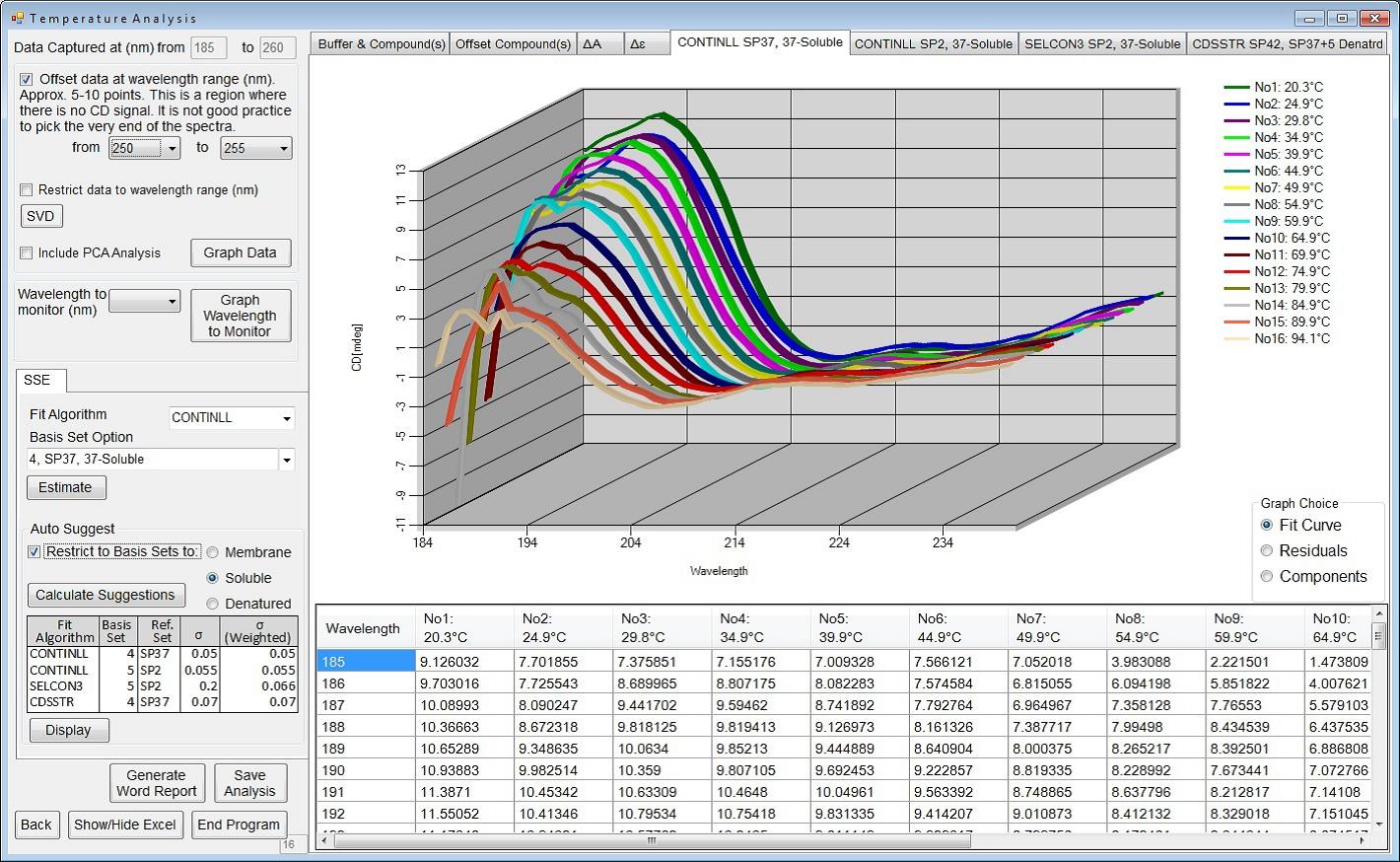
Figure 4: B23 CDApps software showing thermal denaturation data processing feature.

Figure 5: Focus group for the Material Science Workshop held at Diamond on 18 March 2014
Stopped-flow (SF) is a useful tool to study the kinetics of fast reactions such as enzymatic reactions, ligand binding interactions and protein folding/unfolding by mixing the individual components and observing the CD as a function of time. The high photon flux of B23 enables measurements with much better signal-to-noise than that obtained with bench-top CD instruments, as illustrated in Fig. 6
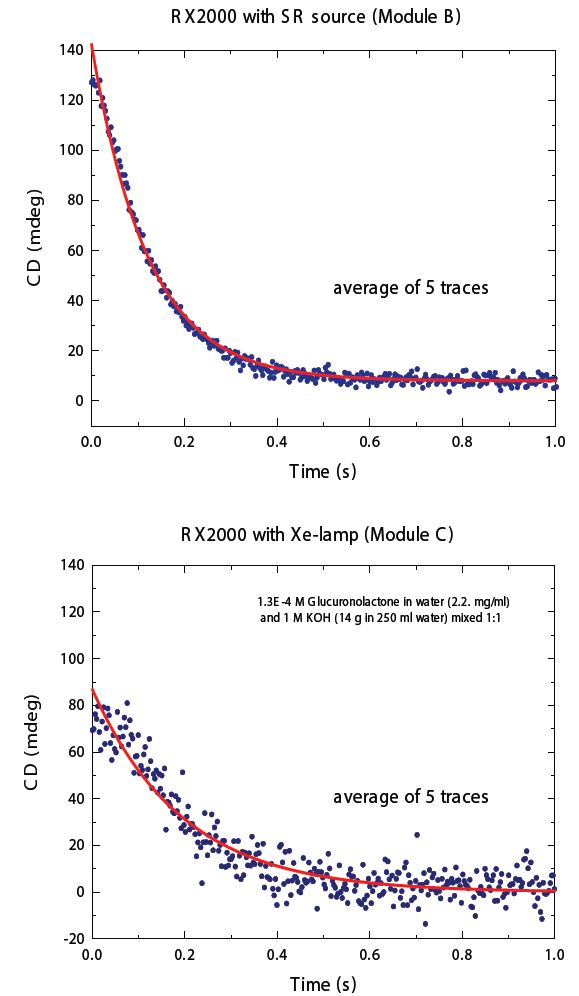
Figure 6: Average of 5 traces of the CD kinetic data of the hydrolysis of glucuronolactone recorded at 225 nm using the same SF apparatus but with different light sources: Xe lamp (Bottom) and B23 module B (Top). The red line is the fitting of the experimental data.
The hydrolysis of glucuronolactone has been used to determine the cell dead-time of the SF attachment of B23. Upon mixing of glucuronolactone with potassium hydrochloride, the superior signal-to-noise ratio data collected at the beamline compared to a benchtop instrument (Fig. 6) was achieved due to the higher photon flux in the far-UV region and the small cross-section of the collimated light beam of B23 at the SF mixing cell. Currently, B23 has two SF attachments: one automated and one manual (Fig. 7).
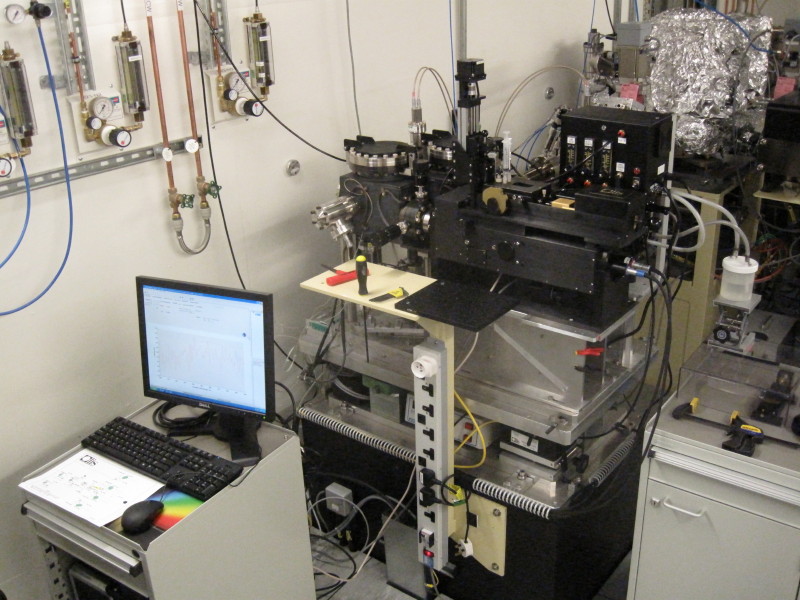

Figure 7: The yellow circles highlight the automated Stopped Flow attachment using Module A (A) and the, manual Stopped-Flow attachment using Module B (B).
After welcoming first users in December 2013, the Solution State SAXS beamline (B21) now operates at full capacity, though some beamline equipment is undergoing commissioning. Over the past year, B21 has provided SAXS capabilities to users throughout the UK and Europe. Samples have included: the components of gene splicing and protein degradation to the RNA polymerase machinery from Flu virus, as well as artificial, self-assembled systems not suitable to cryo-EM (due to size) or X-ray crystallography (due to flexibility).
SAXS is a well-established technique that has developed more slowly than other X-ray methods such as X-ray crystallography. B21 is developing new algorithms and computational approaches for data integration and analysis. In the past, background corrections have always been applied by simply subtracting the SAXS measurement of a sample in the absence of the particles of interest. This method was sufficient when the quality of the X-ray beam and detectors were poor. Now, with the modern capabilities at Diamond, the background corrections must be performed based on the physics of the SAXS experiment. This is leading to the extraction of proper signals in high noise environments. Likewise, the high-throughput capabilities are creating large datasets and B21 is implementing a novel analysis pipeline for data integration and modeling. Already B21 has established more accurate methods for calculating SAXS profiles from atomic models and provides users with the SAXS analysis suite ScÅtter.
I22’s horizontal and vertical bimorph mirrors have returned from repolishing and have improved beam quality significantly, with a threefold improvement observed in vertical beamsize to ~70 μm, depending on focal distance, and improvements to background quality. Components for the Grazing Incidence SAXS (GISAXS) upgrade have begun to arrive. The Symetrie Hexapod has been delivered and is being commissioned offline, and recent visitors to I22 will have seen the new detector platform has been installed. This has capability for independent tube, detector and beamstop motion, improving the flexibility of set up on offer at I22. The large new beamstop vessel contains mechanisation for three beamstops, one for each of direct, reflected and specular beams which will improve greatly the ability to collect high quality GISAXS data. A new L-shaped Pilatus detector for combined 2D SAXS/WAXS has been delivered and will be installed as part of the ongoing GISAXS upgrade.
References:
1. Quaroni L. et al. SR based IR imaging and spectroscopy via FPA on live fibroblasts in D2O enriched medium. BioPhys. Chem. 189 40-48 (2014).
2. Eschenroeder E.C.V. et al. Monitoring the Activation of Copper-Containing Zeotype Catalysts Prepared by Direct Synthesis Using in Situ Synchrotron Infrared Microcrystal Spectroscopy and Complementary Techniques. Chem. Mater. 26 1434−1441 (2014).
The two small angle scattering (SAXS) beamlines I22 and B21 provide methods to interrogate protein conformation and organic structures in the solid and liquid states, whereas the Circular Dichroism (CD) beamline (B23) has been developed to monitor protein secondary structure and interactions. The Multimode InfraRed Imaging and Microspectroscopy (MIRIAM) beamline (B22) implements IR microspectroscopy and imaging of complex biological systems such as cells and tissues of medical interest. As the applications of the Soft Condensed Matter village beamlines have expanded across the Physical Sciences, in recent years specific techniques have developed in niche fields. This is the case of nanoparticle to protein interaction probed by the CD microbeam, in situ gas solid vibrational spectroscopy on catalysts by IR or metal-organic framework (MOF) THz modes, supramolecular assemblies studies by SAXS, and interfaces/surface activity by Grazing Incidence SAXS (GISAXS). A summary of the beamline status, achievements and perspectives in terms of science examples and key technical developments is outlined below and in the Village Developments section further on in this report.
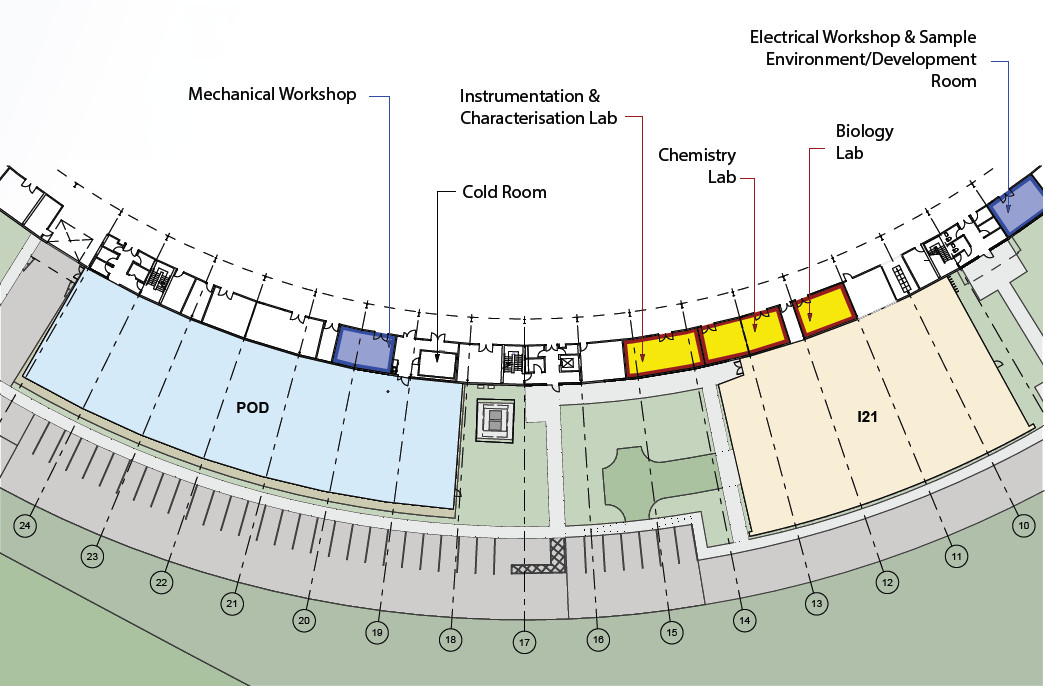
Figure 1: Plan of the new Soft Condensed Matter Village laboratories and workshops located on the periphery of the main synchrotron building
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.