 | ||
Novel hybrid protocells may hint at origins of life |
Many scenarios have been proposed for processes leading to the formation of the protocells that are a pre-requisite to life on early Earth. One theory is based on fatty acid vesicles, which form when naturally occurring fatty acids self-assemble in water to form a bilayer membrane. An alternative theory suggests that protocell formation may occur when organic chemicals and naturally occurring polymers present in aqueous solution spontaneously phase separate to form membrane-free chemically rich liquid microdroplets, called coacervates. Combining these two approaches, a hybrid protocell model has been developed based on the spontaneous self-assembly of a fatty acid membrane on coacervate microdroplets.
Beamline I22 Scientific Highlight
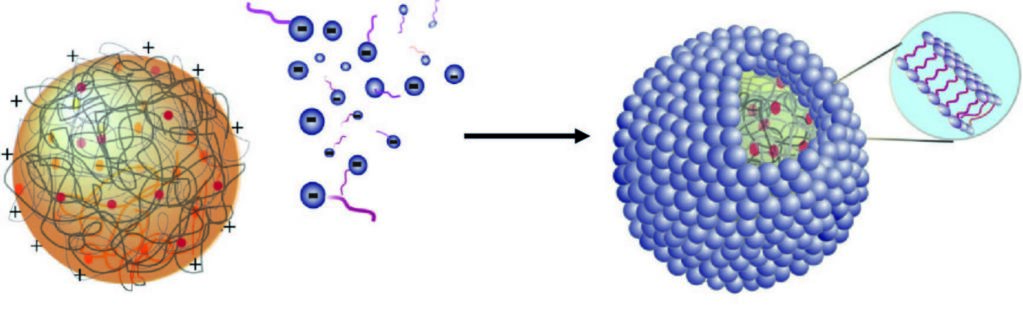
Figure 1: Schematic showing the self- assembly of fatty acid multilayers on the surface of a positively charged coacervate droplet.
Small Angle X-ray Scattering (SAXS) experiments performed using the Small Angle Scattering and Diffraction beamline (I22) were then used in combination with Fluorescence Lifetime Imaging (Fig. 3a), to explore the microstructure of the coated coacervate droplets. The SAXS experiments showed that the fatty acid had assembled to form highly organised multilayers (Fig. 3b) localised on the surface of the coacervate (Fig. 3a). No multilamellar structures were seen in SAXS patterns at low fatty acid concentrations and light scattering experiments confirmed that little to no fatty acid vesicles were present in the dispersion (Fig. 3c). The very high intense X-rays available at Diamond coupled with I22’s extremely sensitive detector were necessary to determine the size and structure of the microcompartments with some key features of interest between 4 and 6 nm in size.
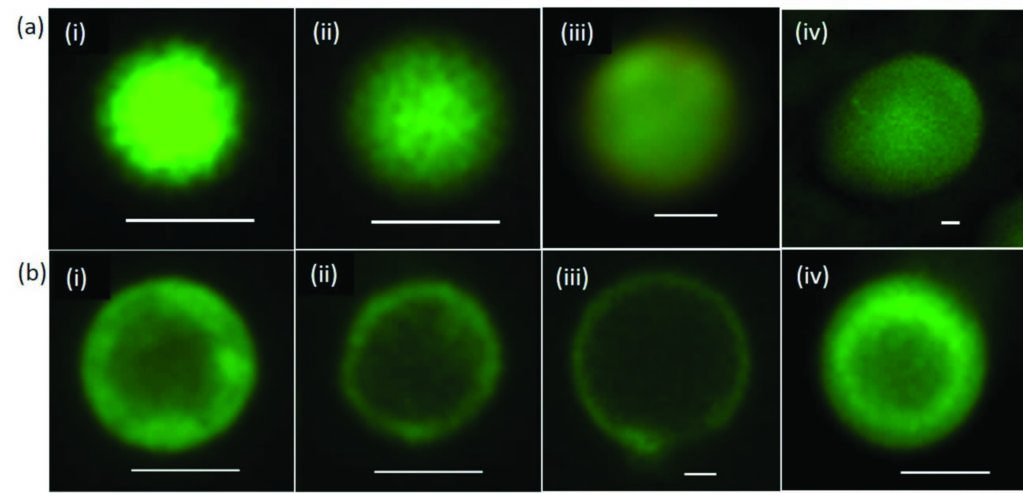
Figure 2: Fatty-acid membrane self-assembly on the surface of positively charged coacervate microdroplets. Fluorescence microscopy images of single droplets stained with the lipid-soluble BODIPY FL C16 dye. (a) Non-coated microdroplets showing homogeneous fluorescence throughout the interior of the microcompartments: (i) polylysine/RNA; (ii) oligolysine/RNA; (iii) oligolysine/ATP; (iv) PDDA/ATP. Scale bars, 1 μm. (b) Fatty acid coated droplets prepared from oleate/polylysine/RNA (i), oleate/oligolysine/ RNA (ii), oleate/oligolysine/ATP (iii) and oleate/ PDDA/ATP (iv) showing assembly of a fatty-acid membrane specifically at the surface of the microcompartments. Scale bars, 1 μm.
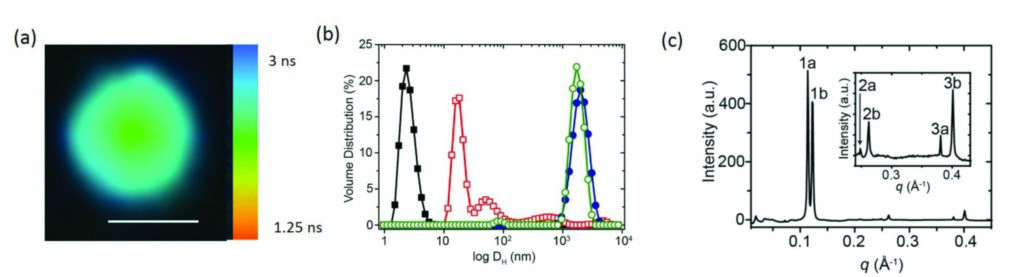
Figure 3: (a) Fluorescence lifetime imaging microscopy image of a fatty acid-coated coacervate droplet containing kiton red showing two different lifetimes and viscosities in the centre (coacervate matrix) and edge regions (lipid membrane); scale bar = 5 μm. (b) Small angle X-ray scattering profile showing Bragg reflections corresponding to a multilamellar fatty acid membrane at the surface of the coacervate droplets. (c) DLS profiles showing volume distributions of hydrodynamic diameters (DH (nm)) for oleate micelles (black filled squares), oleic acid/oleate multilamellar vesicles (red open squares), uncoated PDDA/ATP microdroplets (blue filled circles,) and oleate-coated PDDA/ATP microdroplets (green open circles).Caption with parts indicated where necessary; (a) first part; (b) second part.
Source publication:
Tang, T. Y. D., Hak, C. R. C., Thompson, A. J., Kuimova, M. K., Williams, D. S., Perriman, A. W. & Mann, S. Fatty acid membrane assembly on coacervate microdroplets as a step towards a hybrid protocell model. Nature Chemistry 6, 527-533, doi:10.1038/nchem.1921 (2014).
References:
1. Monnard, P. A. & Deamer, D. W. Membrane self-assembly processes: Steps toward the first cellular life. Anatomical Record 268, 196-207, doi:10.1002/ ar.10154 (2002).
2. Oberholzer, T., Wick, R., Luisi, P. L. & Biebricher, C. K. Enzymatic rna replication in self-reproducing vesicles - an approach to a minimal cell. Biochemical and Biophysical Research Communications 207, 250-257, doi:10.1006/bbrc.1995.1180 (1995).
3. Oparin, A. I. The Origin of Life, 2nd ed. Dover Publications: Dover (1953).
4. Koga, S., Williams, D. S., Perriman, A. W. & Mann, S. Peptide-nucleotide microdroplets as a step towards a membrane-free protocell model. Nature Chemistry 3, 720-724, doi:10.1038/nchem.1110 (2011).
5. Crosby, J. et al. Stabilization and enhanced reactivity of actinorhodin polyketide synthase minimal complex in polymer-nucleotide coacervate droplets. Chemical Communications 48, 11832-11834, doi:10.1039/ c2cc36533b (2012).
Funding acknowledgements:
We thank the Engineering and Physical Sciences Research Council (EPSRC, UK) and European Research Council (Advanced Grant) for financial support. We thank the EPSRC for a Career Acceleration Fellowship (grant number EP/E038980/1) to M.K.K. and the Malaysian government for the award of a PhD studentship to C.R.C.H. We acknowledge beamline I22 (N. Terrill and A. Smith) at Diamond Light Source, W. Briscoe for beamtime and R. Richardson and M. Thomas for assistance and use of software for X-ray analysis.
Corresponding author:
Professor Stephen Mann, University of Bristol, s.mann@bristol.ac.uk


 A brighter light for science
A brighter light for science
