___________________________________
Industrial Liaison Group:
Tel: +44 (0) 1235 778797
E-mail: [email protected]

Nuclear energy for electricity generation offers large-scale low-carbon energy production but suffers with challenges such as the handling of radioactive waste materials. Therefore, the proper management of spent fuel arising from nuclear power production is a key issue for the sustainable development of nuclear energy

Various advanced processes for used fuel reprocessing have been established over the last decade or two. Of these, aqueous separations processes using solvent extraction technology remain the leading option. The PUREX process is a chemical method used to purify fuel for nuclear reactors or nuclear weapons. It is an acronym standing for Plutonium Uranium Redox EXtraction. PUREX is the de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used ("spent", or "depleted") nuclear fuel. Additionally, much effort has been placed on methods to recover the minor actinides for conversion into another form and to create alternative aqueous processes to replace PUREX; for example, the “GANEX” (Grouped Actinide Extraction) process which aims to co-recover all actinides in a single process.

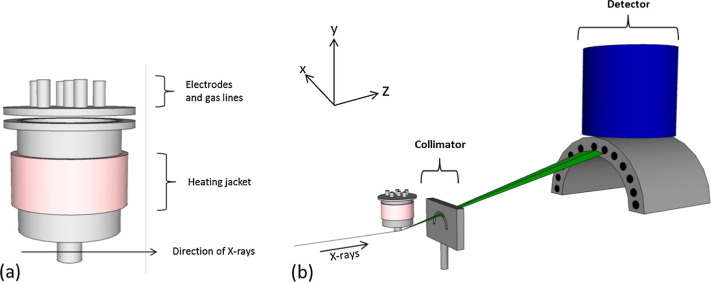
An aluminium electrochemical cell was used to house the molten salt, and electrodes were custom built for this investigation. The electrochemical and EDXD measurements were taken at 450 °C and the results confirm that the electrochemical reduction of uranium dioxide to uranium metal seems to occur in a single, 4-electron-step, process. The rate of formation of α-uranium is seen to decrease during electrolysis and could be a result of a build-up of oxygen anions in the molten salt. Slow transport of O2− ions away from the UO2 working electrode could impede the electrochemical reduction.
Further exploration of the microstructure of working electrodes will therefore be the focus of future work.
Read the full paper here....
Following the electroreduction of uranium dioxide to uranium in LiCl–KCl eutectic in situ using synchrotron radiation L.D. Brown, R. Abdulaziz, R. Jervis, V.J. Bharath, R.C. Atwood, C. Reinhard, L.D. Connor, S.J.R. Simons, D. Inman, D.J.L. Brett, P.R. Shearing. Journal of Nuclear Materials Volume 464, September 2015, Pages 256–262. http://dx.doi.org/10.1016/j.jnucmat.2015.04.037
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.