___________________________________
Industrial Liaison Group:
Tel: +44 (0) 1235 778797
E-mail: [email protected]
What would the world be like without nucleation? To give just a few examples, there would be no “ice” in an ice cream, no “fizz” in a fizzy drink, no clouds, no volcanic eruptions, no gemstones, no seashells and no drugs. Whenever crystals, bubbles, droplets, melts or minerals are formed, the process begins with a small number of atoms or molecules coming together to form an embryonic form of the new phase. The term nucleation refers to this very first step in a phase transition or structural reorganisation. For example, the formation of a snowflake is preceded by nucleation of embryonic ice crystals (solid phase) from water vapour (gas phase), usually on salt or dust particles that act as seeds from which the new phase can grow. Such seeded nucleation, scientifically referred to as heterogeneous nucleation, represents the vast majority of nucleation phenomena in nature. In the absence of seeds, nucleation often does not proceed readily. For example, ultrapure water in a container with very smooth surfaces can be cooled to temperatures far below 0°C without forming any ice crystals. The moment such undercooled liquid water is poured onto a material with a rough surface (clay, for example) nucleation takes place and ice forms instantaneously.
The key to understanding and controlling nucleation lies in understanding how the initial molecular assembly is “triggered”. Generally, these early nucleation stages are poorly understood, as they can be challenging to characterise experimentally. Experimental detection of nuclei is restricted by the size of the nuclei, which typically have sizes on the scale of nanometers. Moreover, movement of such small objects is highly random, making them hard to trace, and they are present only in very small numbers and short-lived, making their detection even more of a challenge. It is also of interest to understand the molecular structure of the phase from which nucleation takes place.
Influencing what is happening in this pre-nucleation stage could enable us to accelerate or slow down a nucleation process, and to control the properties of the product formed, such as the size and shape of crystals as well as their internal molecular structure. Such understanding may enable improvements in the strength of a material or design better drug delivery routes by improving solubility. Recent scientific studies have thus addressed nucleation events during the formation of proteins, minerals, crystals of small organic molecules and metals from solution. Major research effort in this area of science is driven by its relevance to a vast range of practical applications such as in healthcare, water treatment, solar energy, energy storage and high value manufacturing of, for example, fine chemicals and formulations of foods, feeds and consumer products.
Building on fundamental research developed under an EPSRC-NSF grant, researchers from Diamond Light Source, the University of Manchester and Synchrotron SOLEIL aimed to probe the nucleation of metal nanoparticles. Time resolved spectroscopy at the B18 beamline at Diamond and at ODE of Synchrotron Soleil allowed the team to probe the nucleation of Palladium (Pd) metal from a solution of a Pd salt. Crucially, the time-resolved spectroscopic facilities enabled them to follow the structural, chemical and compositional changes in situ at an unprecedented level that no routine home laboratory techniques can provide. Most importantly, they were able to capture and characterise the pre-nucleation state, which is a metastable equilibrium state, in which the chemical composition of Pd was observed to randomly fluctuate between the reactants (the ions of the dissolved Pd salt) and unstable nucleation products (nanoparticles of Pd metal) on a time scale of minutes. In this pre-nucleation equilibrium, the existence of which was theoretically predicted already more than 70 years ago, the chemical driving force is sufficient to enable the assembly of unstable metal nanoparticles but insufficient to facilitate their spontaneous growth to stable metal particles.
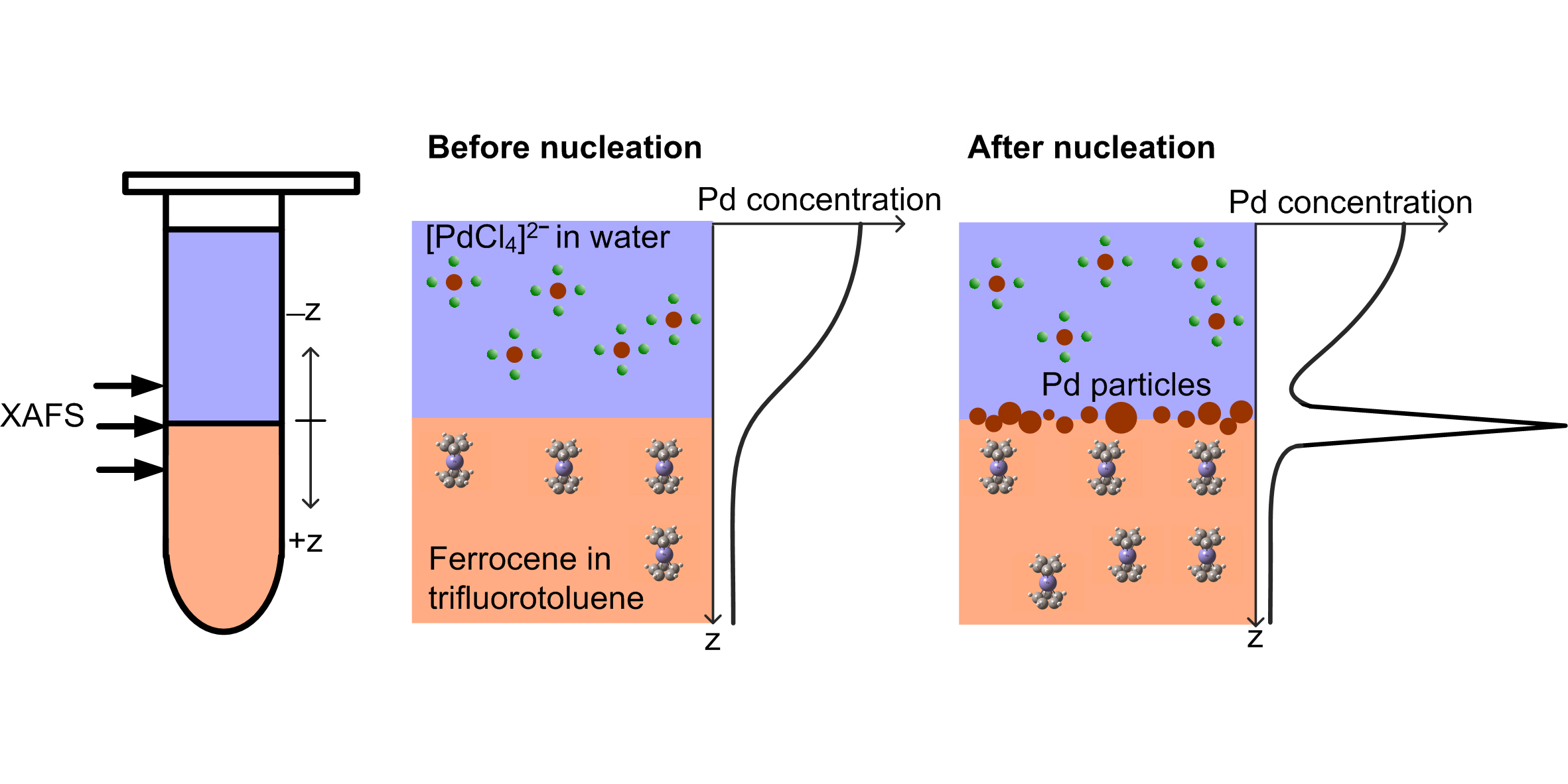
Image courtesy of ©CrystEngComm
![A solution of [PdCl4]2−(aq)](/dam/jcr:944856e5-b104-49c3-ad8e-b4a259a235ec/Tetrachloropalladate_ions_in_aqueous_solution.jpg)
In order to observe these nuclei the researchers investigated a reaction that occurred only at the interface formed by contacting two immiscible solutions. An aqueous solution containing the Pd salt (palladium chloride) was brought in contact with an organic solvent containing the reducing agent that facilitated the formation of the metal. Use of such a liquid-liquid system limits the reaction to the interface between the two fluids, where the reactants can come into contact.
Controlling the concentrations of the reactants in the adjacent phases permits adjustment of the driving force (technically called supersaturation) for nucleation and thus preparation, and potentially stabilisation, of the metastable pre-nucleation state. The liquid-liquid interface also confines the location of the nucleation reaction, thus simplifying the spectroscopic experiment and improving spectroscopic contrast when compared to a bulk phase reaction.
It is believed that this study represents the first step towards a better understanding of metallic nucleation, and the observed behaviour may generally be applicable to other reactive nucleation processes as well. Building on the study, this collaboration has been awarded more beamtime on B18 and I20 at the Diamond Light Source as well as on ROCK at Synchrotron Soleil where the researchers are looking to use external electrochemical control (instead of mere chemical control) to drive the nucleation process, thereby enabling in situ variations and more precise control of the process.
The combination of liquid-liquid interface and time-resolved spectroscopy is not only useful when applied to the study of metal nanoparticles, it may also be useful to study organic or biological systems using soft X-ray spectroscopies/imaging, with relevance for food, cosmetics, consumer products and pharmaceutical industries.
Detection and Characterisation of Sub-Critical Nuclei during Reactive Pd Metal Nucleation by X-ray Absorption Spectroscopy
S.-Y. Chang, Y. Gründer, S. G. Booth, L. B. Molleta, A. Uehara, J. F. W. Mosselmans, G. Cibin, V.-T. Pham, L. Nataf, R. A. W. Dryfe & S. L. M. Schroeder
CrystEngComm 18 (2016) 674-682.
DOI: 10.1039/C5CE01883H
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.