____________________________________
Industrial Liaison Group:
Tel: +44 (0) 1235 778797
E-mail: [email protected]
The COVID-19 virus pandemic caused by the SARS-CoV-2 coronavirus poses an ongoing serious global health threat that requires effective and safe therapeutics. The SARS-CoV-2 coronavirus uses its spike glycoprotein to bind to the host receptor angiotensin-converting enzyme-2 (ACE-2) and enter the body’s cells.
The receptor-binding domain (RBD) of the spike glycoprotein is the main target for neutralising antibodies as it can block the virus-host interaction and prevent the infection. The RBD is highly variable and can mutate to escape recognition by antibodies, thus reducing their efficacy and increasing the risk of resistance.
Moreover, the RBD is only exposed transiently on the spike glycoprotein, making it difficult for antibodies to access and bind to it. Novel antibodies are needed that can recognise and neutralise the RBD of SARS-CoV-2 with high potency and stability, regardless of its variability and accessibility
Conventional antibodies have been used as therapeutic agents against various infectious diseases, but they have limitations such as high production costs, low stability, and immunogenicity.
Heavy chain–only antibodies (HCAbs) are a unique type of antibodies found in the Camelidae family that are composed of heavy chains and lack light chains and the CH1 domain. Nanobodies are the smallest functional units of HCAbs that can bind to antigens with high affinity and specificity, and offer advantages of reduced sizes, and higher stabilities than conventional antibodies.
A llama-derived single-domain nanobody, C5, has high COVID-19 neutralisation potency. C5 binds strongly to the RBD of the SARS-CoV-2 coronavirus spike glycoprotein, even across different variants, preventing the virus from attaching to ACE-2 proteins in the body and allowing the infection to enter human cells. Characterisation of the binding of C5 to the RBD is essential to the further development of effective therapeutics.
To enhance the therapeutic potential of C5, scientists from the Rosalind Franklin Institute constructed a fusion protein C5-Fc comprising two C5 domains attached to a glycosylated Fc region of a human IgG1 antibody.
Small angle X-ray scattering (SAXS), in both batch and SEC-SAXS modes, were used by scientists at University College London, in combination with small angle neutron scattering (SANS), to characterise the solution structure of C5-Fc and its interaction with the viral receptor-binding domain (RBD).
The results showed that C5-Fc has reduced size and increased stability compared to conventional antibodies, facilitating its production, storage, and delivery. This may also confer its broad-spectrum activity against different SARS-CoV-2 variants and compatibility with the human immune system.
Combining synchrotron SAXS and SANS data allowed scientists to determine the solution structure in vivo of the C5-Fc protein, This approach provided a simple and fast method to unveil information about the size, shape and conformation of C5-Fc in solution, which is relevant for its biological function and stability. The solution structure of C5-Fc shows this is able to neutralise the virus. It also enabled the team to uncover and remove the effect of any aggregates or impurities in the sample which can affect its quality and efficacy. The results have led to a greater understanding of therapeutic activity of the C5-Fc protein and its role in preventing COVID-19 viral infection.

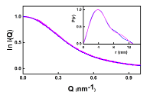
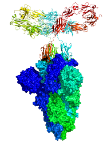

The project benefitted from ample protein supplies and the excellent B21 instrument at Diamond. When we compared our C5-Fc solution structure with the prior crystal structure of the C5-RBD complex, we showed that three nanobodies (see image 3 - green, cyan, and orange ribbons) were capable of binding to a single virus spike simultaneously to neutralise this. Thus Diamond gave us a novel insight into this interaction.
Prof. Stephen Perkins, University College London.

Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.