In work recently published in PNAS an international team of researchers characterised an important degradation step, allowing the breakage of lignin that leads to the production of individual components, which can be further harvested. To do so, they utilise several Diamond Light Source instruments: the I23, I03 and B21 beamlines.
Compared to animals, plants don’t have a bony skeleton. They rely on rigid cell walls that separate each plant cell. These cell walls are composed of cellulose, pectin and lignin, making these molecules among the most abundant on earth. Lignin is a hydrophobic compound and plays a crucial role in vascular tissue, making them impermeable and allowing the transport of water in the plant efficiently. Lignin is a huge and complex molecule composed of different precursors called monolignols. The composition of lignin varies among plants.
From an industrial perspective, lignin is well known in the paper industry because it represents a third of the mass of the paper precursor. Lignin is a coloured component that yellows in the air and needs to be removed to have white paper. Currently there is only limited use for lignin and it is burned as low value fuel in these industries. New research and development have improved the transformation of lignin into value added components (biofuels, chemical compounds…) but research is still needed to improve the degradation process of lignin. A way to harvest these components is through enzymatic degradation. In work recently published in PNAS an international team of researchers characterised an important degradation step, allowing the breakage of lignin that leads to the production of individual components, which can be further harvested. To do so, they utilise several Diamond Light Source instruments: the I23, I03 and B21 beamlines.
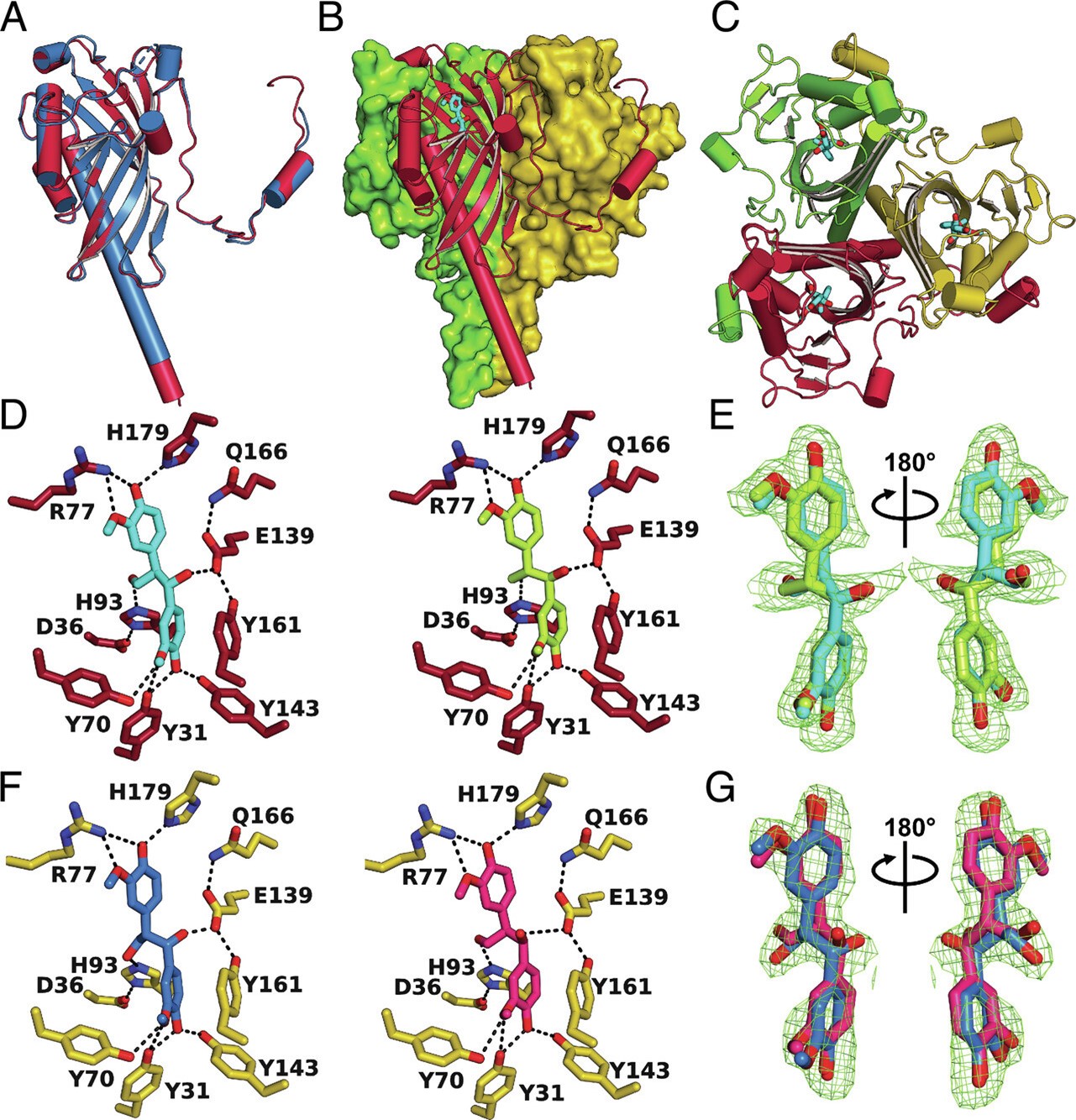
Lignin is a heterogeneous, recalcitrant aromatic polymer. This complexity is derived from the various bonds by which different lignin monomers are linked together. Nevertheless, bacteria have evolved enzymes that are fine-tuned for cleaving the specific bonds to enable utilisation of lignin-derived carbon. In this study, researchers performed biochemical, structural, and mechanistic characterisation of two bacterial enzymes, LdpA from N. aromaticivorans and a homolog protein, Sphingobium sp. SYK-6. LdpA belongs to the Nuclear Transport Factor 2 (NTF-2) protein family, which is known to have some enzymatic activities as dehydratase and decarboxylase. Both bacteria were known for their capacity to open aromatic compounds.
To perform the structural characterisation of both proteins, the researchers used two different MX beamlines, I03 and I23. Structural characterisation of the protein in complex with its ligand allowed them to clearly determine where the interactions take place between the protein and the substrate. Further SAXS experiments performed at B21 confirmed that LdpA forms homotrimers and is stable at 25°C. Thanks to this structural information, they were able to perform specific mutagenesis experiments on LdpA to verify the activity of 7 important residues needed for the catalytic activity.
Dr Kuatsjah, main author of this article explained:
Excellent support provided by the beamline researchers enabled high quality data for our various structure determination needs.
Dr Armin Wagner, Principal Beamline Scientist I23 added:
It’s been a pleasure to be part of this study. Understanding how enzymes like LdpA work is an important step towards plant-based chemistry to reduce the dependency on petroleum.
This publication is interesting as it shows that LdpA has the capacity to degrade lignin-derived aromatic compounds linked by a so-called β–1 bond, which is a strong carbon–carbon bond. This is the first time that such an activity is characterised for a member of the NFT-2 family. These findings provide a platform for future enzyme engineering and microbial engineering to expand the amount of lignin that is available for conversion into high-value biochemicals.
To find out more about the I23 beamline or discuss potential applications, please contact Principal Beamline Scientist Armin Wagner: [email protected]
To find out more about the B21 beamline or discuss potential applications, please contact Principal Beamline Scientist Nathan Cowieson: [email protected]
Kuatsjah E, et al. Biochemical and structural characterization of a sphingomonad diarylpropane lyase for cofactorless deformylation DOI: 10.1073/pnas.2212246120
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.