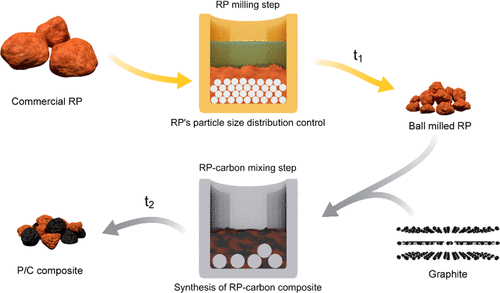
"Reducing the particle size leads to lower forces on the particles and fewer cracks. How much do we need to reduce the particle size? That is the fundamental question."
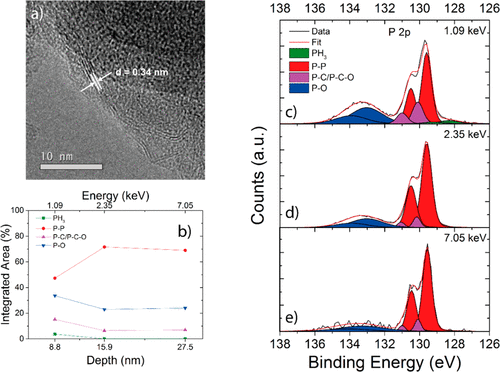
"The results from Diamond showed what we had expected to see - more phosphorus-carbon bonds on the surface, and an increase of phosphorus-phosphorus bonds as we sampled the bulk, confirming the presence of a carbon coating."
The results of this research showed that an RP particle size of below two microns offers better capacity and a significantly longer life cycle for sodium-ion batteries. The team were able to confirm that their process led to the successful formation of a carbon coating and that longer milling times lead to a more uniform layer. These are important steps towards the development of high-capacity anodes for SIBs, but also apply to other battery chemistries.
The next step in this research is a more fundamental study to investigate why this is the critical particle size, why it prevents cracks, and whether cracking can be prevented in another way.
To find out more about the I09 beamline, or to discuss potential applications, please contact Principal Beamline Scientist Tien-Lin Lee [email protected].
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.