
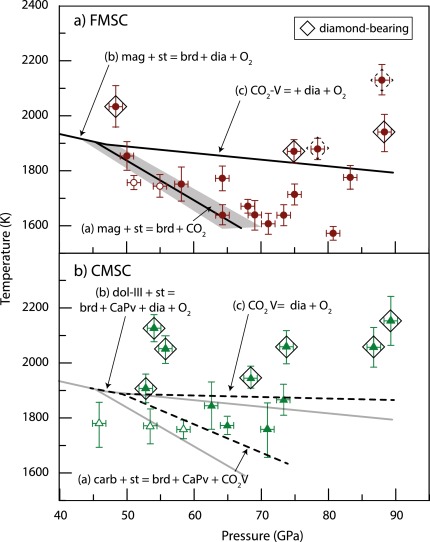
The global carbon cycle is key to Earth’s habitability, giving our planet a stable and hospitable climate, and an atmosphere relatively low in carbon dioxide. Textbook diagrams of the carbon cycle typically show carbon repositories in the atmosphere, the oceans, and the soil, but not what’s going on deeper into the planet. Earth’s mantle, which holds at least 75% of the planet’s total volume, potentially holds more carbon than all of the other reservoirs combined. Uniquely in the solar system, Earth has active plate tectonics, which move materials between the mantle and the crust. However, investigating the mantle is not easy. It extends from about 35 to 2,890 km below the surface, with temperatures which rise from 200 °C at the upper boundary with the crust to approximately 4,000 °C at the core. A team of experimental geoscientists from the University of Bristol has used diamond anvil cells (DAC) and laser heating to recreate the extreme conditions in Earth’s mantle. Their research, published in Earth and Planetary Science Letters, investigated what happens to carbonate minerals when they are subducted from the oceanic crust into the mantle. They found that decarbonation reactions prevent subduction of carbonate deeper than around 1500 km, and that the mantle stores carbon in the form of diamond.
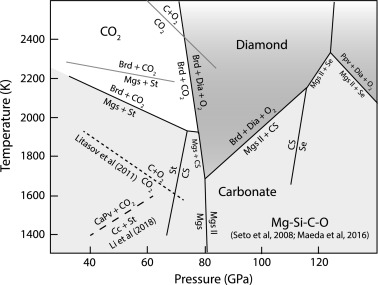
Conditions in the mantle are extreme, and samples from this region are rarely found on the surface. Most of the carbon that is subducted into the mantle is returned to the surface via volcanic activity. However, analysis of mined diamonds shows that about 1% are formed much deeper in the mantle than is normal, at depths of up to 800 km. The carbon in these ‘superdeep’ diamonds comes from Earth’s surface, telling us that some of the subducted carbon is stored, and transformed, in the mantle.
Lead author Dr James Drewitt explains:
"Eventually these super-deep diamonds could be returned to the surface in upwelling mantle plumes, and this process could represent one of the sources of super-deep diamonds that we find at the surface and which provide the only direct evidence we have of the composition of the deep earth."
Dr Drewitt and his team are regular visitors to Diamond. He says:
"To generate extreme high pressures in a diamond anvil cell, our samples are very small, approximately 50 microns in diameter. Synchrotron facilities like Diamond generate the very bright, high-energy and highly focused X-ray beams that we need to analyse the bulk atomic-scale structure of these tiny samples, synthesised under extreme conditions, using X-ray diffraction. It’s fantastic to have a synchrotron essentially on our doorstep. We can literally drive down with the samples, and it makes it very easy to collaborate closely with the beamline staff."
The next step for this research is to combine these high pressure and high-temperature experiments with advanced computer simulation techniques to analyse other mantle materials. Dr Drewitt says:
There is potentially several ocean's worth of water transported deep into the mantle, and when released this will induce melting of Earth's upper and lower mantle. At the moment we can’t adequately test or understand current models of the dynamic behaviour of this water rich molten rock because we do not know their composition or their physical properties. Further experiments at extreme conditions including the in-situ high-pressure laster heating facilities in commissioning at beamline I15, plus the advanced computer simulations that we are currently working on will help to resolve these problems.
Drewitt, JW et al. The fate of carbonate in oceanic crust subducted into earth's lower mantle. Earth and Planetary Science Letters, 511, 213-222 (2019). DOI: 10.1016/j.epsl.2019.01.041.
Anzellini, S et al. Laser-heating system for high-pressure X-ray diffraction at the Extreme Conditions beamline I15 at Diamond Light Source. Journal of Synchroton Radiation, vol 25 (2018). DOI: 10.1107/S1600577518013383
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.