We are one step further to uncovering a new way to stave off dengue fever thanks to important work carried out at the I02 beamline at Diamond Light Source. The study, recently published in Nature Immunology, describes how an antibody effectively targets the dengue virus.
Dengue virus affects hundreds of millions of people worldwide and is an untreatable infection. Secondary infections with dengue can lead to a life-threatening form of the disease due to a phenomenon called antibody-dependent enhancement (ADE). Additionally, efforts to develop a vaccine against the virus have been hindered by ADE.
A huge collaborative effort sought to investigate ADE in dengue, and two antibodies were characterised that bound to the envelope protein of the dengue virus. One of the antibodies was found to be a potent neutraliser of the virus, but importantly was unable to promote ADE.
Macromolecular crystallography at Diamond detailed the way in which the antibodies interacted with the viral protein, and the team saw that the ability to promote ADE was related to the orientation of an antibody on the surface of the protein.
The knowledge gained from the study could provide the groundwork to develop vaccines that do not evoke an ADE, and might lead to a successful dengue vaccine.
The dengue virus is transmitted by mosquitoes and is widespread in Southeast Asia, South America and the West Pacific regions. Although usually a mild and transient infection, some people can experience a severe form known as dengue haemorrhagic fever, which can be fatal. Despite the risk posed by the virus, there is no existing vaccine or treatment.
One of the reasons for the lack of a successful vaccine is the existence of multiple serotypes of the virus. In fact, the cause of dengue haemorrhagic fever is via a second infection with a different serotype. Scientists believe that dengue haemorrhagic fever is due to ADE, whereby pre-existing antibodies to the virus serve to encourage the viral infection. ADE is thought to have thwarted a potential dengue vaccine, so understanding how this unfavourable mechanism can be overcome is paramount to fighting the disease.
Scientists hailing from the University of Oxford, Mahidol University in Bangkok, Chiang Mai University and Diamond set out to characterise neutralising antibodies against the dengue virus and understand their effects on ADE.
The team were initially drawn to a mouse antibody against the dengue virus, known as 3H5, because preliminary experiments hinted that it had some unusual properties.
The group compared how 3H5 behaved in the presence of dengue serotypes versus another anti-dengue antibody called 2C8, which served as a reference. They carried out enhancement cell-based assays and saw that while the 2C8 antibody elicited a classical ADE response, 3H5 did not.
Dr Max Renner, post-doctoral scientist at the Division of Structural Biology, University of Oxford and joint author of the study explained their next steps:
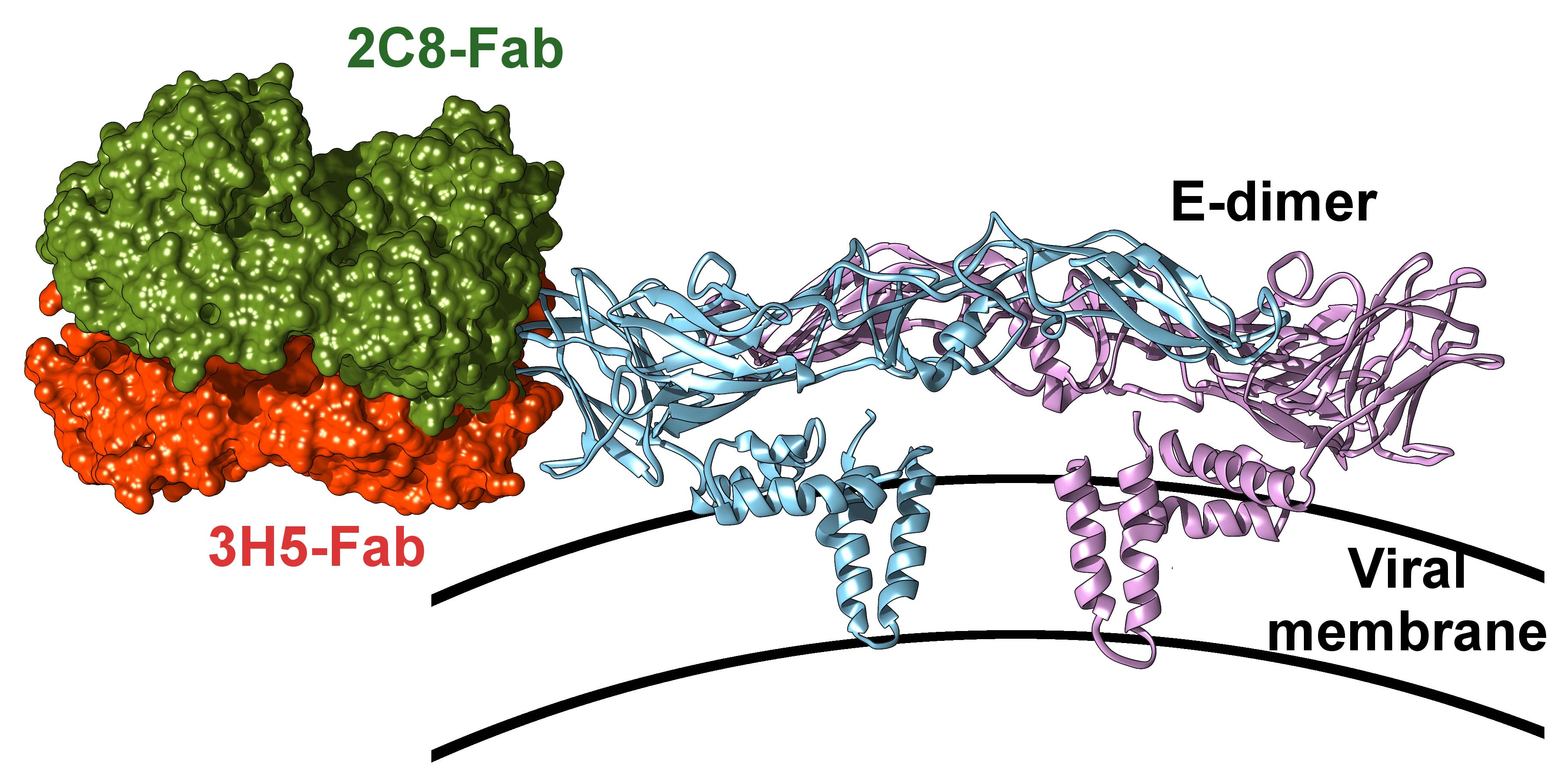
To figure out exactly where the antibodies bound the virus, we used crystallised complexes. We saw the antibodies bound to the exposed part of the virus, known as the envelope protein domain III (EDIII).
The crystal structures obtained at the I02 beamline at Diamond allowed the scientists to see the exact conformation of the antibodies on the dengue virus. Dr Renner explained,
We saw that 3H5 bound to an additional region of the EDIII, which caused it to adopt a different orientation on the virus surface to 2C8. This meant that 3H5 was in a flat orientation with its Fc regions hidden from Fc receptors
Cryo-EM was conducted at the University of Oxford to confirm their findings. They saw 2C8 antibodies projecting out of the viral surface and ready to interact with the immune system to promote ADE, whereas flattened 3H5 was inaccessible to the immune system.
“It is exciting how the orientation of an antibody binding to the surface of a virus can potentially affect how well Fc receptors can bind the antibody and affect the capability of ADE,” concluded Dr Renner.
These groundbreaking findings could pave the way to a successful dengue vaccine that does not encourage ADE, and the team are continuing to look for more antibodies that might have therapeutic potential. Moreover, the structural insights gleaned from the study could help design safer vaccines against other viruses.
Our former I02 beamline, referenced in this research, has been upgraded to the VMXi beamline, which is a fully automated facility for characterisation of, and data collection directly from, crystallisation experiments in situ.
For those interested in research like the above, and to find our more about VMXi, or to discuss potential applications, please contact please contact Senior Beamline Scientist Juan Sanchez-Weatherby: [email protected].
Renner M et al. Characterization of a potent and highly unusual minimally enhancing antibody directed against dengue virus. Nature Immunology 2018.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.