The properties of nanocrystalline materials, where crystallinity is limited to particles of a few nanometres in dimension, are heavily researched in a number of contemporary areas. This is because once the crystal is reduced to such a small size, the properties may change dramatically from the bulk material. The structure of the surface atomic arrangement also becomes a significant proportion of the total structure, which may change the reactivity of the material in a nanocrystalline form. Examples of such behaviour are now well known in terms of optical and electronic properties of confined matter, and also in catalysis where selective activity towards particular substrates may be tuned by selecting which crystal face is presented. In the further development of nanocrystalline materials we have been studying the use of soft chemical approaches for the preparation of complex oxides, containing two or more metals in a precise ratio, with control over particle size and morphology. Part of this work has involved characterisation using B18, the Core EXAFS beamline at Diamond Light Source, and here we describe some of the first results obtained using this facility.
We have focused on cerium dioxide (CeO2) nanomaterials. CeO2 itself has well established uses in catalysis where it provides a redox–active support that allows control of oxygen concentration in important applications such as automotive catalytic converters, where pollutant gases such as nitrogen oxides and carbon monoxide are transformed into more benign emissions1 . The reactivity of CeO2 arises largely from its oxygen storage capacity, a property associated with considerable oxide ion mobility in the solid-state, and the ability of Ce to interconvert rapidly and reversibly between the +4 and +3 oxidation states at moderate temperatures1, and also gives use of the material in solid-oxide fuel cell materials. Other more recent applications of CeO2 include as a catalyst support for precious metals in the water gas shift reaction, used for the purification of hydrogen for fuel cell applications2, as abrasive agents for chemical mechanical planarisation of integrated circuits3, and recently as scavengers for superoxide radicals in potential medical applications4. Often the activity of CeO2 is optimised when nanocrystalline forms of the material are used3. In further tuning the properties in these diverse applications, chemical control over small amounts of dopant metal ions within each nanocrystal is an important strategy.
We have used hydrothermal synthesis, where chemical precursors are heated in an aqueous medium above the boiling point of water to bring about the one-step crystallisation of metal oxide materials5. The advantage of this approach lies in the use of a solvent to bring about the reaction rather than on the high temperature solid-state reactions usually associated with the preparation of ceramic oxides. Thus control of crystal form may prove possible, and also by judicious choice of chemical reagents, control of the oxidation state of constituent metals may be achieved. We thus investigated the preparation of doped cerium oxide materials by the use of CeIIICl3.7H2O in the presence of sodium bismuthate, NaBiVO3, as an oxidising reagent. Transmission-electron microscopy images (Fig. 1), of the samples prepared by this route reveal the presence of highly crystalline nanoparticles with facetted surfaces and typical particle diameters of less than 10 nm, the precise value depending on the Bi content.

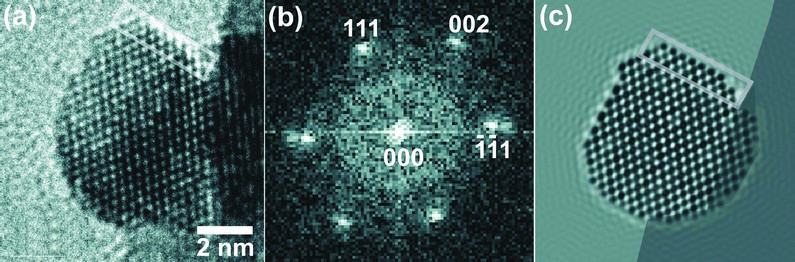
Figure 1: High-resolution TEM of a crystallite of Ce0.5Bi0.5O1.75 (a) is an original image with one facet highlighted , (b) an indexed FFT obtained from the lattice image and (c) a multislice image simulation produced for a similar fragment as in (a) with the same facet highlighted.
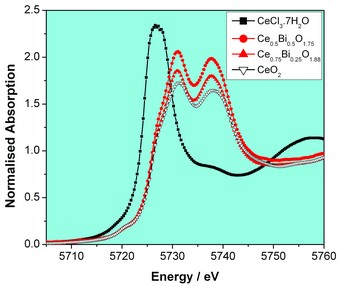
Structural characterisation of nanocrystalline materials is complicated by the fact that the classical Bragg diffraction from small particles is broadened considerably. Therefore a variety of characterisation methods must be used to build up a complete picture of their structure. For the Bi-doped CeO2 particles we have achieved this by using a set of complementary techniques at both Diamond Light Source and the ISIS neutron facility. Thus neutron scattering measurements provided radial distribution functions that can be modelled using crystal-structure based models. In order to proceed with this modelling, XAFS measurements on B18 were vital to examine the local coordination environment of each metal and to establish the oxidation states of the two metals. Figs. 2 and 3 show plots of XANES spectra measured at the Bi LIII-edge and the Ce LIII-edge, along with those of suitable reference materials. These spectra provide a fingerprint of local atomic structure that is difficult to obtain using other methods, such as XPS, which is surface sensitive. At the Bi LIII-edge (Fig. 2) we see no evidence for the presence of Bi(V), since this is characterised by a pre-edge 2p3/2 – 6s transition, present in the highest oxidation state where the 6s levels are unoccupied, as seen in the reference material NaBiO3. Thus we can prove that the nanomaterials contain only Bi(III) resulting from the reduction of the Bi in the chemical precursor, although the local Bi environment is different to that in a-Bi2O3. In contrast at the Ce LIII-edge (Fig. 3) the spectra show that our materials contain only Ce(IV), by comparison to the Ce(IV) reference material CeO2 that shows a characteristic ‘double hump’, ascribed to transitions from 2p3/2 to valence 5d-like states. The XANES spectra confirm the strategy used in our chemical synthesis with the NaBiO3 acting as an oxidant to yield CeO2 doped with Bi(III). We have gone on to develop detailed structural models for our materials involving the partial replacement of Ce by Bi in CeO2, which include local distortion of Bi(III) ions from the ideal cubic metal site in the fluorite lattice.
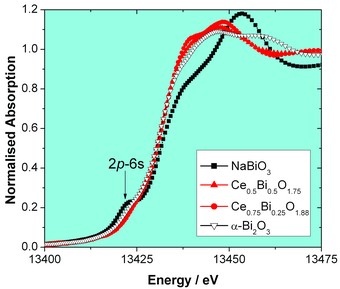

Figure 2: Bi LIII-edge XANES of two nanocrystalline Bi-doped ceria samples and spectra from the two reference material NaBiO3 and α-Bi2O3.
In terms of properties, our Bi-doped CeO2 materials are readily reduced under a flow of dilute hydrogen (in a temperature programmed reduction experiment, for example). This is a diagnostic test of the catalytic activity for various applications, since it is one measure of the ease with which oxide ions may be removed from the solid lattice. The reducibilty of our samples occurs more readily than pure CeO2 and can be explained by their distorted structures as well as the presence of oxide vacancies, proven from the structural work. We also assessed the materials’ stability using time-resolved X-ray diffraction under reactive gas flow, and this shows that at high levels of Bi doping the materials are susceptible to phase separation leading to collapse. Thus in future work this must be overcome to provide robust nanomaterials with high activities.
Figure 3: Ce LIII-edge XANES of of two nanocrystalline Bi-doped ceria samples and spectra from the two reference material CeO2 and CeCl3.7H2O.
In ongoing work we are developing our synthetic strategy to target more complex materials with unusual combinations of metal oxidation states: this will allow us to tune properties in a rational manner. As well as using static XAFS measurements, as described here, we will also make use of in-situ studies where we hope to exploit ongoing developments at Diamond to measure data rapidly under reaction conditions in order to examine the activity and stability of various nanocrystalline materials.
Sardar, K., Playford, H.Y., Darton, R.J., Barney, E.R, Hannon, A.C., Thompsett, D., Fisher, J., Kashtiban, R.J., Sloan, J., Ramos, S., Cibin, G. and Walton, R.I., Nanocrystalline Cerium Bismuth Oxides: Synthesis, Structural Characterisation and Redox Properties. Chemistry of Materials Vol 22, 6191-6201 (2010)
References
- Trovarelli, A. Catalysis by Ceria and Related Materials. Imperial College Press, London (2002).
- Fu, Q. et al. Science. 301, 935 (2003).
- Feng, X.D. et al. Science. 312, 1504 (2006).
- A. Karakoti, A. et al. Chem. Soc. Rev. 39, 4422 (2010).
- Modeshia, D.R. & Walton, R.I. Chem. Soc Rev. 39, 4303 (2010)..
Funding Acknowledgement
Engineering and Physical Sciences Research Council, UK and Johnson Matthey plc.