Copper ions form an important part of everyday life, as they are incorporated into a number of proteins and enzymes, amongst which are some that help the body to respire, fight infections and maintain health bones and tissues. Understanding how these ions are accommodated in a solution of water is key to understanding many of these processes.
Scientists working at the Diamond Light Source and the ISIS Facility may have answered a controversial question at the heart of the chemistry of copper. The research has shown that the hydration structure of the copper ion (Cu2+) may be more tetrahedral than octrahedral, helping to explain previously observed results.
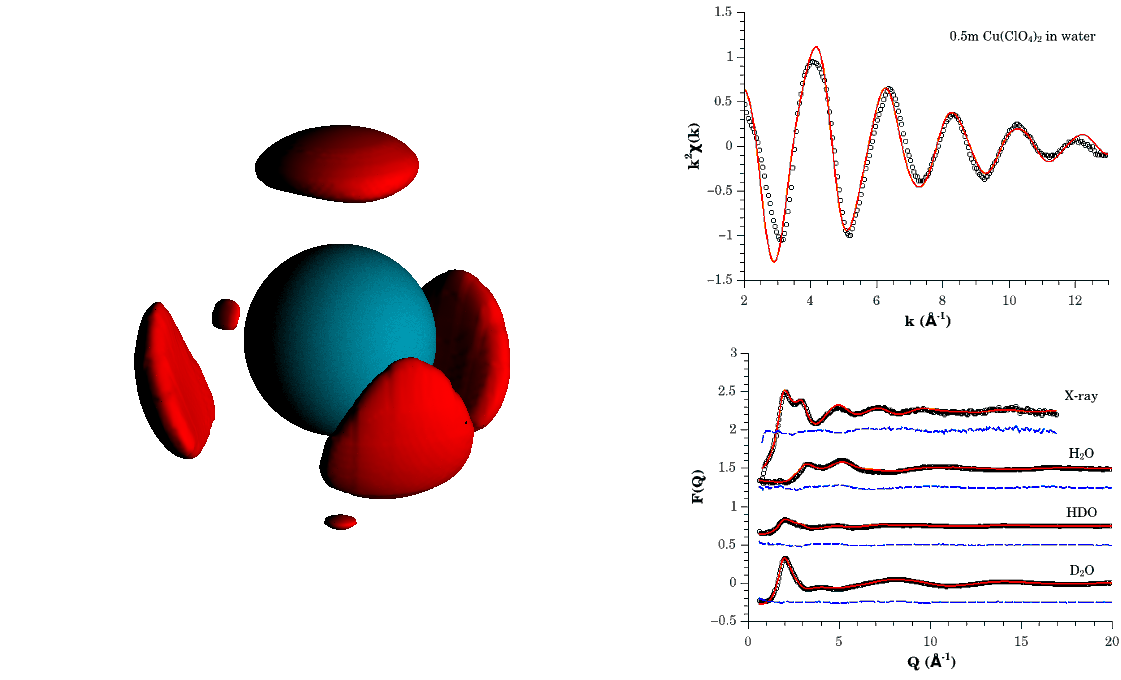
Spatial density plot highlighting the highest probability regions of finding water molecules around the Cu(II) aqua-ion. This function was derived from a simultaneous refinement of neutron and x-ray diffraction, and x-ray absorption spectroscopy data shown in the right hand panel.
Previous research in this area had proved controversial, and had brought about a number of new studies to determine how many water molecules each copper ion bound to. In this study, the researchers from Diamond, Rutherford Appleton laboratories and the Universidad de Zaragoza used three methods to investigate the hydration structure. Neutron scattering was performed at the ISIS Facility to build up a wider picture of the water bound to the copper ions. X-ray diffraction using a standard bench top diffractometer helped the researchers build up the intermediate structure, and X-ray absorption spectroscopy was performed on beam line I20 at the Diamond Light Source to build up fine detail at the local copper sites.
Bringing all this data together allowed the researchers to building up a comprehensive model of the hydration structure of Cu2+.
Extended X-ray Fine Structure (EXAFS) and X-ray Absorption Near Edge Structure (XANES) spectroscopy data were obtained using the scanning branch on the beam line I20. The experimental data matched models obtained using the Empirical Potential Structure Refinement (EPSR) technique, which was adopted to minimise potential bias in the results in favour of any pre-conceived outcomes.
This publication is the first paper to result from measurements performed on the scanning branch of beamline I20. Principal Beamline Scientist Sofia Diaz-Moreno says, “These first results are really exciting for the beamline and demonstrate its utility in the area of solution chemistry. We are now working to optimize the beamline to probe atomic environments in extremely low concentrations of elements, which is required for meeting the challenges of real world situations, for example in catalysts, polluted environments or at physiological conditions.”
The hydration structure of Cu2+: More tetrahedral than octahedral? Daniel Bowron , Monica Amboage , Roberto Boada Romero , Adam Freeman , Shusaku Hayama , Sofia Diaz-Moreno RSC Advances DOI: 10.1039/c3ra42400f
 Spatial density plot highlighting the highest probability regions of finding water molecules around the Cu(II) aqua-ion. This function was derived from a simultaneous refinement of neutron and x-ray diffraction, and x-ray absorption spectroscopy data shown in the right hand panel.
Spatial density plot highlighting the highest probability regions of finding water molecules around the Cu(II) aqua-ion. This function was derived from a simultaneous refinement of neutron and x-ray diffraction, and x-ray absorption spectroscopy data shown in the right hand panel.