Shigella flexneri is a bacterium which causes dysentery, resulting in a million deaths worldwide every year. To infect humans, it uses a complex molecular machine which attaches itself to a host cell and uses a needle to pierce the cell membrane and deliver proteins that hijack cellular processes to facilitate infection. Scientists from the University of Oxford have been using Diamond to study how the machine identifies a host cell and triggers the transfer of proteins. This work is important for understanding not only Shigella flexneri but the processes of many bacterial infections.
In detail
The type 3 secretion system (T3SS) is used by many Gram-negative pathogenic bacteria, including Shigella flexneri, to transfer virulence proteins into host cells. Initially the bacterium must assemble the external needle and identify a host cell . In the next stage, translocator proteins insert into the host cell membrane, which then triggers the export of effector proteins enabling them to enter the host cell directly. For this process to be effective, the pathogen must ensure that the proteins for assembling the needle are secreted first, and that the effector proteins must not be secreted until contact has been made with the host cell. Therefore the pathogen must have both temporal and hierarchical control over the secretion mechanism.
The exact mechanisms that underlie this process are not fully understood, but one proposal is that a protein (known as MxiC in Shigella flexneri) acts as a physical block to the secretion apparatus. Despite variation across bacterial species, a distinct family of proteins has been identified that could provide this mechanism. Knocking out members of this family has no effect on needle formation, but significantly reduces translocator proteins, and in some species results in enhanced secretion of effector proteins.
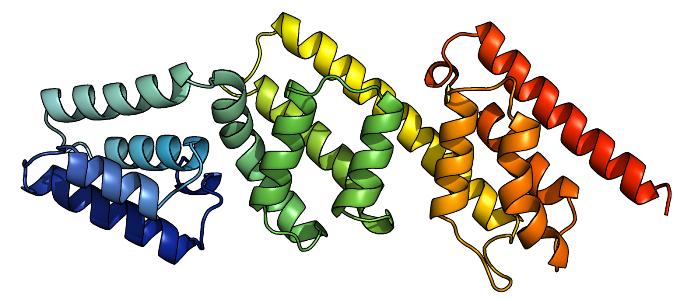
A coloured ribbon diagram of MxiC, from blue at the N terminus to red at the C terminus.
However, there are several differences between members of this family across species. Janet Deane and colleagues from Susan Lea's group at the University of Oxford used synchrotron X-rays to determine and refine the structure of the MxiC protein in Shigella flexneri and compared this to the equivalent protein in the Yersinia species. The results confirmed the homologous nature of the two proteins, and suggested a mechanism for binding the various components of the T3SS.
"We are only just beginning to gain insight into these complex systems. Facilities such as Diamond are essential for our further work."Susan Lea, University of Oxford
Janet E. Deane, Pietro Roversi, Carole King, Steven Johnson and Susan M. Lea, Structures of the Shigella flexneri Type 3 Secretion System Protein MxiC Reveal Conformational Variability Amongst Homologues, J. Mol. Biol. (2008) 377, 985–992
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.