All living things propagate through the process of cell division. Histone proteins play a vital role in this complex process, but the exact molecular mechanisms that make it possible are not well understood. Histones act as spools for DNA, allowing it to wind around them to make compact packages that fit neatly into cells. Scientists from the European Molecular Biology Laboratory in Heidelberg, Germany, have been studying how histones interact with a chaperone protein to answer the fundamental question of how histones package our genome. This work has been published in the Proceedings of the National Academy of Sciences (PNAS).
In Detail
The FACT complex facilitates chromatic transcription, and also plays a role as a histone chaperone. However, the molecular mechanisms that underpin these actions remain elusive. The group from the European Molecular Biology Laboratory has been studying the FACT subunit Spt16 in Schizosaccharomyces pombe, to analyse its biochemical, structural and mutational behaviour. They discovered the existence of a peptidase-like domain which directly binds the H3 and H4 histones. These interactions may help our understanding of FACT-mediated nucleosome reorganization events.
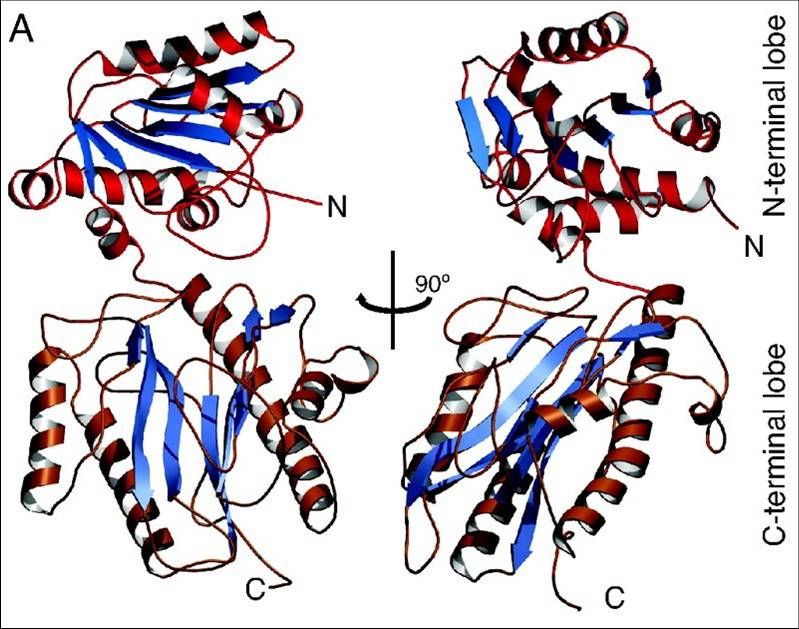
Fig. 1. Structure of the aminopeptidase domain of Spt16. (A) View of the N-terminal domain (residues 1–442) of S. pombe FACT subunit Spt16 in two orientations. The module consists of an N-terminal lobe (Upper) and a larger C-terminal lobe (Lower) that is structurally related to aminopeptidases. Courtesy of PNAS.
"FACT has now been studied for many years. Biochemical and genetic data have shown an important role for it in remodeling histones on DNA during the process of transcription, as well as during the replication of our DNA. We knew that this protein complex binds to histones H2A and H2B, so we surmised that it might only be involved in chaperoning H2A/H2B dimers. Our discovery that the peptidase domain also binds H3/H4 histones suggests that this histone chaperone indeed may engage with all the four core histones that are required for DNA packaging in our cells. Thanks to the structure we obtained at Diamond, we are now able to dissect FACT's molecular mechanism in exciting detail."Andreas Ladurner, EMBL
Stuwe T, Hothorn M, Lejeune E, Rybin V, Bortfeld M, Scheffzek K and Ladurner L The FACT Spt16 "peptidase" domain is a histone H3-H4 binding module, PNAS July 1, 2008 vol. 105 no. 26 8884–8889
http://www.pnas.org/content/105/26/8884
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.