During normal healthy cell division, the cell goes through a series of checkpoints to prevent abnormal or damaged cells from proceeding with division. But if one of these checkpoints is defective, chromosomal instability can result, leading to the growth of malignant cells. Scientists from the University of Manchester have been using Diamond to study a protein called Mps1, which regulates the number of chromosomes during the cell cycle, making it a potential target for new cancer treatments.
In detail
Chromosomal instability can result from defective control of checkpoints and is associated with malignant cell growth. Monopolar spindle 1 (Mps1) is a dual-specificity protein kinase that has important roles in the prevention of aneuploidy during the cell cycle, and might therefore be a potential target for new therapeutic agents in the treatment of cancer.
To gain insights into the molecular mechanism of Mps1 inhibition by small molecules, the groups led by Dr Patrick Eyers and Dr. Lydia Tabernero from the University of Manchester used Diamond to determine the X-ray structure of Mps1, both alone and in complex with the ATP competitive inhibitor SP600125. Mps1 adopts a classic protein kinase fold, with the inhibitor sitting in the ATP-binding site where it is stabilized by hydrophobic interactions. They identified a secondary pocket, not utilised by SP600125, which might be exploited for the rational design of specific Mps1 inhibitors. These structures provide important insights into the interaction of this protein kinase with small molecules and suggest potential mechanisms for Mps1 regulation.
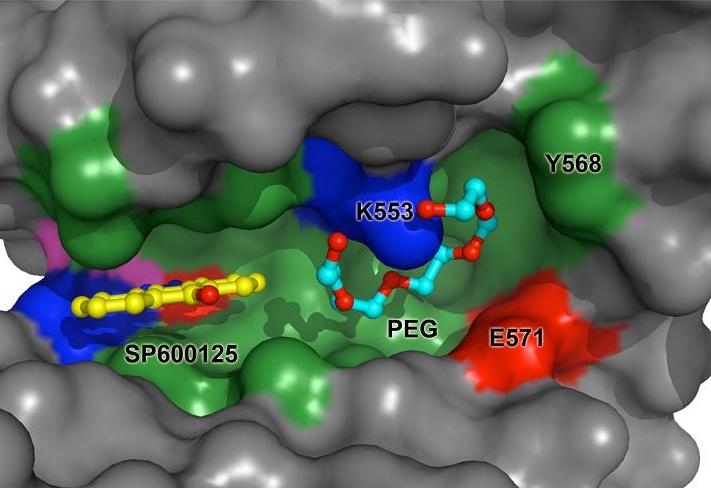
Surface representation of the SP600125 binding site in human Mps1. A molecule of PEG from the crystallisation solution is bound in a secondary pocket next to the catalytic Lys553.
"This work presents the first crystallographic structure of human Mps1, an important regulator of chromosomal stability and a potential target in cancer therapy. Our research has revealed several important structural features and additional binding sites that could be exploited for the development of specific Mps1 inhibitors."Dr Eyers and Dr Tabernero, University of Manchester
Matthew L.H. Chu, Leonard M.G. Chavas, Kenneth T. Douglas, Patrick A. Eyers and Lydia Tabernero, Crystal structure of the catalytic domain of the mitotic checkpoint kinase Mps1 in complex with SP600125, J. Biol. Chem
doi: 10.1074/jbc.M803026200
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.