Find out more about our ambitious upgrade project, delivering more brightness, more coherence, and greater speed of analysis to UK science. More about Diamond-II
![]()
Find out more about Diamond's response to virus research.
![]()
When we want to store or dispose of radioactive waste, or clean-up nuclear sites and mines, it's essential to understand how uranium interacts with, and moves through, the environment. This interaction is primarily controlled by the oxidation state of the uranium, with U(VI) relatively mobile in the environment, and U(IV) mainly immobile. However, biogeochemical reactions (a complex set of interactions between dissolved species, mineral surfaces and biological activity) can alter the oxidation state of uranium, and hence its mobility in the environment.
This picture is complicated further in contaminated land and geodisposal systems, where interactions with iron and sulfide minerals are important factors in controlling uranium mobility. Previous studies have shown that transformations of iron (oxyhydr)oxide minerals result in significant impacts on the oxidation state and mobility of uranium.
While uranium-sulfide compounds have not been identified under environmentally relevant conditions, confirming their presence provides a significant new insight into our understanding of uranium biogeochemistry. More generally, the UK has a substantial nuclear legacy which needs to be cleaned up and managed, and this work demonstrates the deep understanding we can develop of these complex systems. In turn, this knowledge underpins efforts to clean-up and manage radioactive wastes and develops highly skilled people to work on these nationally important challenges.

A new paper to be published on 16 December provides a significant new insight into our understanding of uranium biogeochemistry and could help with the UK’s nuclear legacy.
Conducted by a team of researchers from the University of Manchester, Diamond Light Source and Radioactive Waste Management, their work shows for the first time how uranium forms a uranium-sulfur complex under conditions generally found in the environment and how this compound can be an important intermediary in uranium immobilisation. Published in Environmental Science & Technology, the paper is called "Formation of a U(VI)-persulfide complex during environmentally relevant sulfidation of iron (oxyhydr)oxides"1.
Professor Katherine Morris, Associate Dean for Research Facilities in the Faculty of Science and Engineering, University of Manchester and the Research Director for the BNFL Research Centre in Radwaste Disposal explains why recreating and studying these chemical complexes is highly relevant for understanding and dealing with radioactive waste. She explains:
To be able to predict the behaviour of the uranium during geological disposal, we need to take into account that it may have interacted with other processes taking place in the ground. These so-called biogeochemical reactions are often a complex set of interactions between dissolved chemical species, mineral surfaces, and microorganisms.
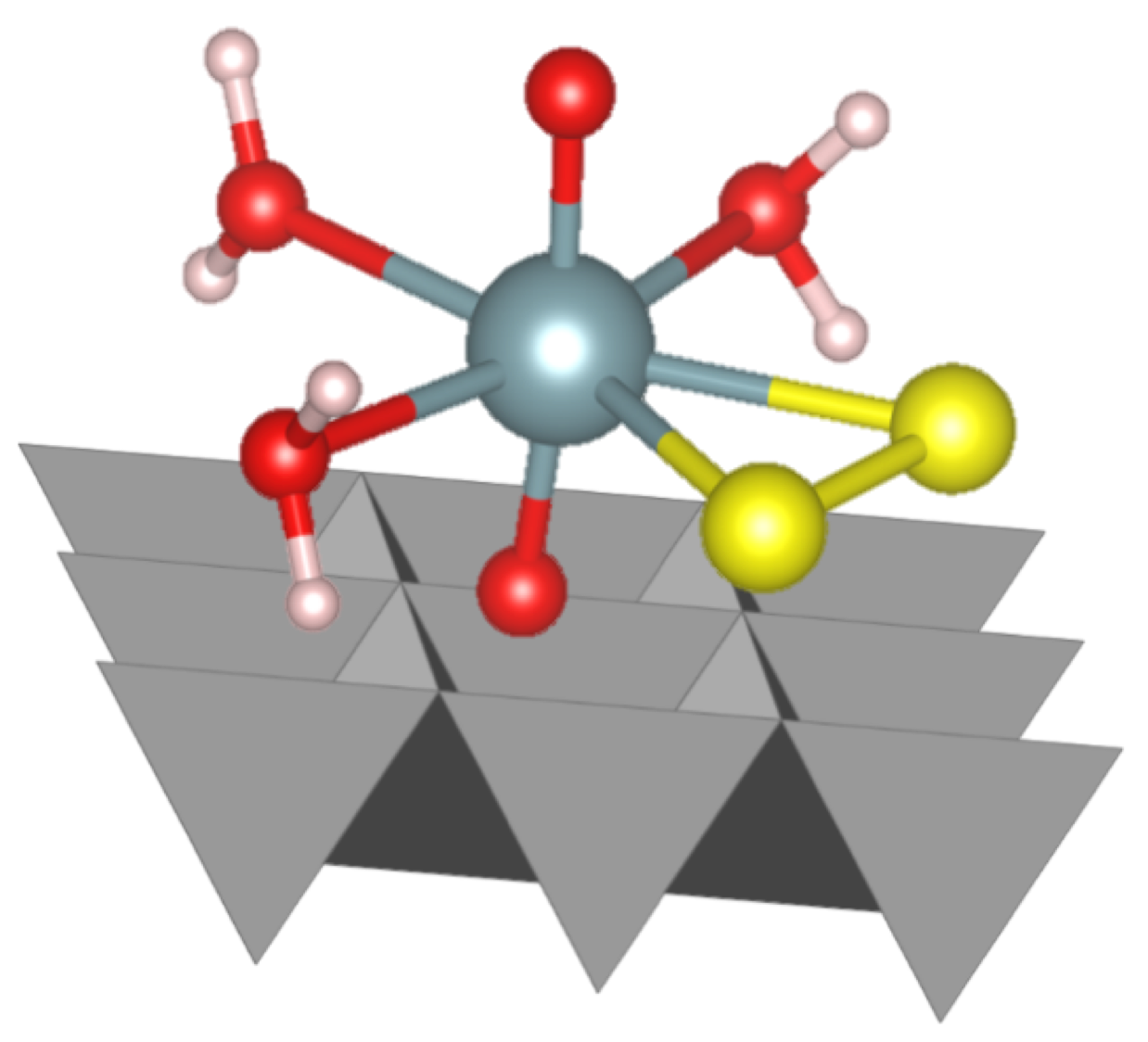
Shining the synchrotron beam onto the sample causes the uranium within to emit X-rays. By analysing the X-ray signal from the samples our team were able to determine the chemical form of uranium, and to which other elements it is bound. To further validate the theory on the formation pathway of the uranium-sulfur complexes, our team also made computer simulations to conclude which type of complex is more likely to form. This is the first observation of this form of uranium under aqueous conditions, and provides new insight into how uranium behaves in environments where sulfide is present. This work demonstrates the deep understanding we can develop of these complex systems and this knowledge will help underpin efforts to manage radioactive wastes in a geological disposal facility.
Dr Luke Townsend, Postdoctoral Fellow in Environmental Radiochemistry at The University of Manchester, who undertook this research as part of his PhD further adds:
When trying to mimic environmental processes in the laboratory, it’s a challenge to produce accurate, high quality, reproducible science with such complex experiments, whilst also maintaining relevance to the geodisposal environment. However, obtaining exciting results such as these makes all the hard work and commitment to the project from myself and the group, both in our labs in Manchester and on the beamlines at Diamond, completely worthwhile.
The XAS measurements were performed at Diamond on beamlines I20 and B18 by the researchers who used highly controlled sulfidation experiments that mimic biogeochemical processes in the deep underground environment. This was combined with geochemical analyses and computational modelling to track and understand uranium behaviour.
Physical Science Director at Diamond, Laurent Chapon concludes;
This is another example of how Diamond’s state of the art analytical tools are enabling scientists to follow complex processes and help them to tackle 21st century challenges. In this instance, our beamlines allowed the users to gain real insight into the environmental relevance of this new uranium-sulfur complex, which feeds into our understanding of geological disposal.
1 http://dx.doi.org/10.1021/acs.est.9b03180 - available online from 06:01 16th December 2019.
The paper is called "Formation of a U(VI)-persulfide complex during environmentally relevant sulfidation of iron (oxyhydr)oxides" (http://dx.doi.org/10.1021/acs.est.9b03180) and the authors, from the University of Manchester, Diamond Light Source and Radioactive Waste Management, are: Luke Townsend, Samuel Shaw; Naomi Ofili, Nikolas Kaltsoyannis ; Alex Walton, J. Frederick W. Mosselmans; Thomas Neill, Jonathan Lloyd; Sarah Heath; Rosemary Hibberd; Katherine Morris.
The work is funded by EPSRC and Radioactive Waste Management and was performed by Luke Townsend and the team using I20 and B18 beamlines at Diamond.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.