Keep up to date with the latest research and developments from Diamond. Sign up for news on our scientific output, facility updates and plans for the future.
Related publication: Griffith K. J., Wiaderek K. M., Cibin G., Marbella L. E. & Grey C. P. Niobium tungsten oxides for high-rate lithium-ion energy storage. Nature 559, 556–563 (2018). DOI: 10.1038/s41586-018-0347-0
The increasing energy storage needs of electric vehicles and mobile devices is driving research into higher performance batteries. One particular challenge for some applications is safely charging/discharging fast enough, and most materials that offer both high power and fast charging require nanoparticles that are expensive to make and difficult to produce in large quantities.
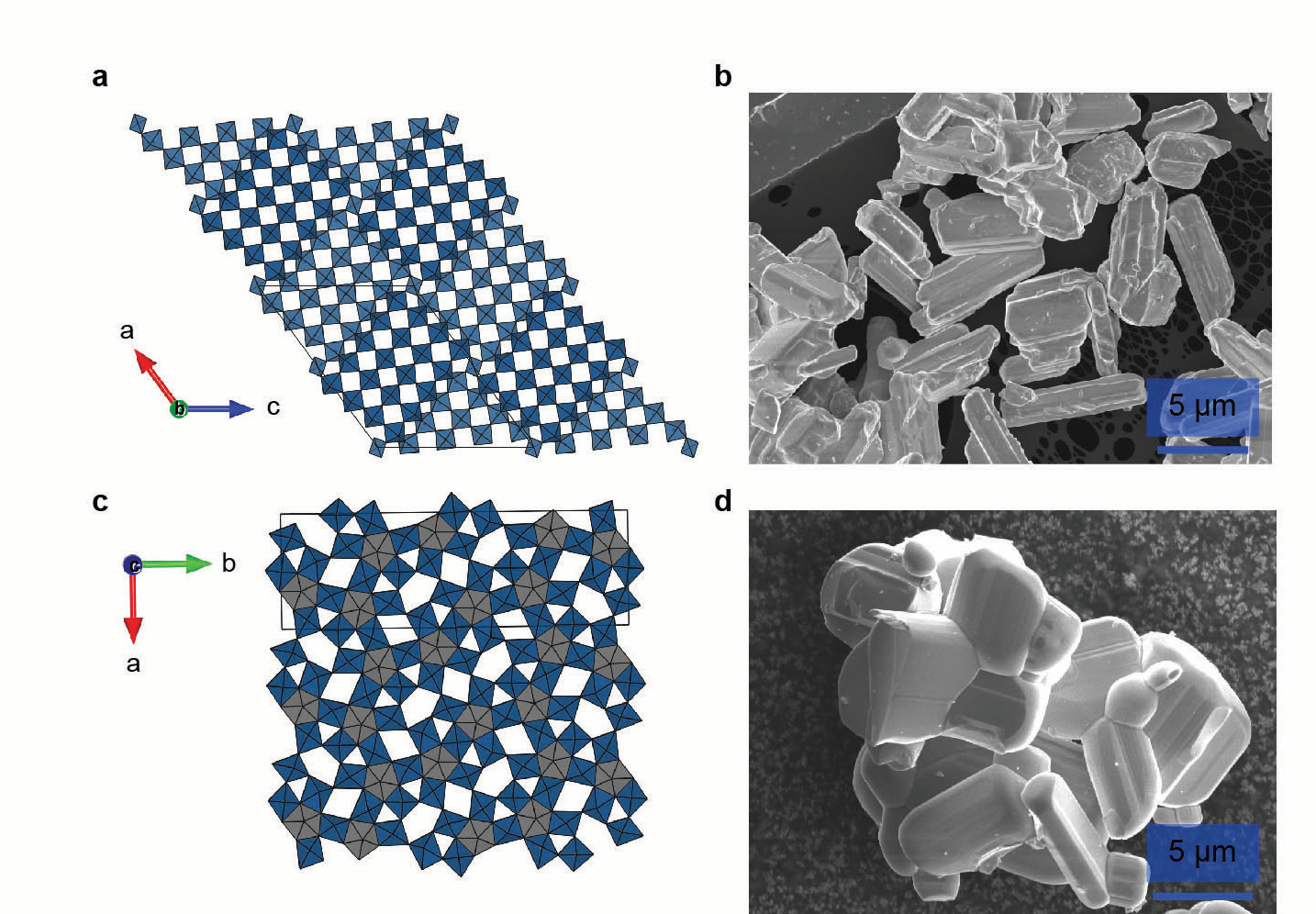
Advanced batteries are required as the world moves toward electric vehicles, intermittent renewable energy sources, and increasingly power-intensive portable electronics and ‘smart’ devices. Modern lithium-ion batteries contain a positive electrode made of a metal oxide and a negative electrode made of graphite. For safety reasons, the use of graphite limits how fast these can recharge or how much power they can deliver. A new negative electrode, in combination with a standard positive electrode, could enable a high-performance lithium-ion battery that can recharge in just a few minutes, depending on the size of the device, which would facilitate new technologies and accelerate the transition away from fossil fuels.
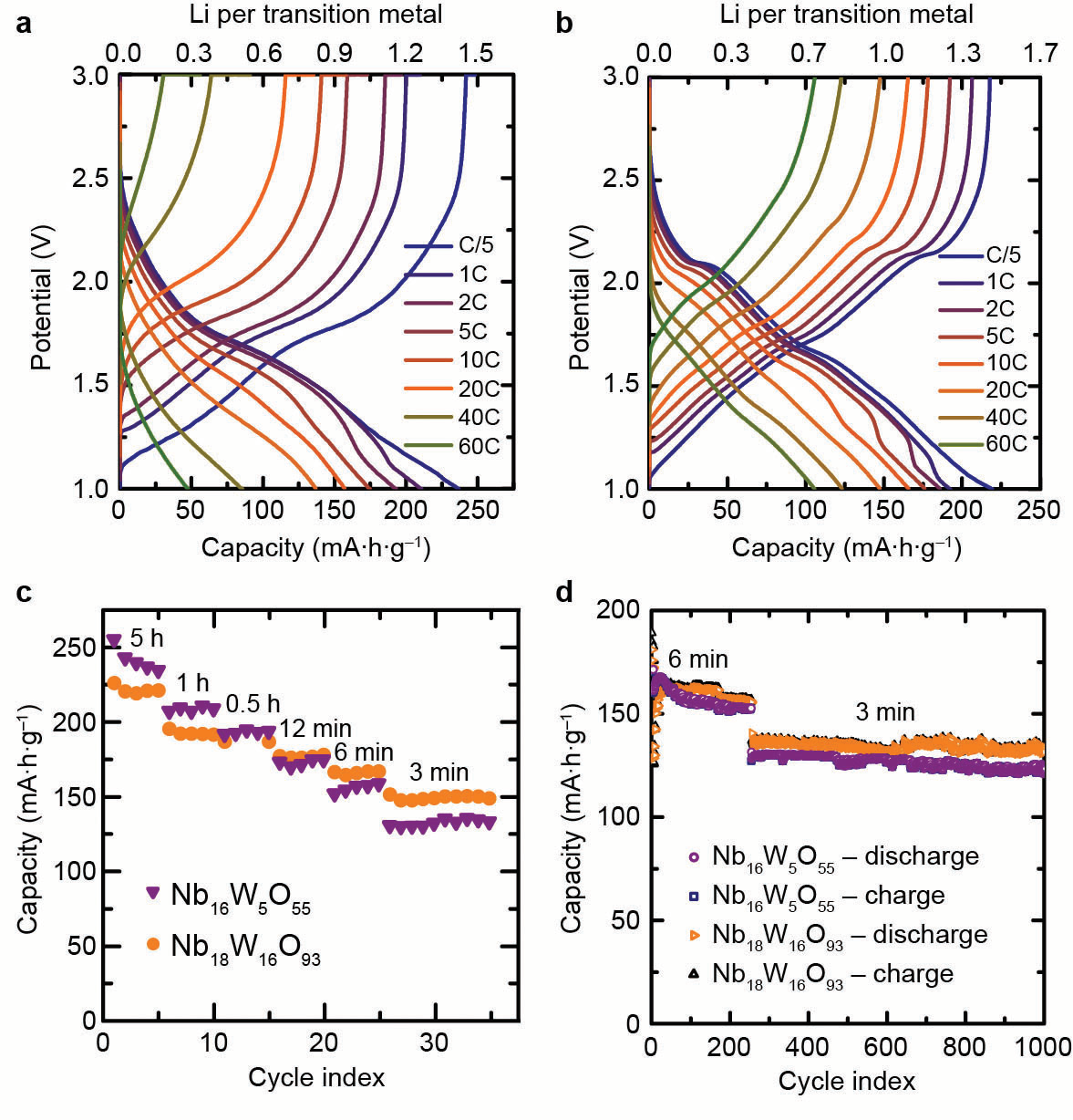
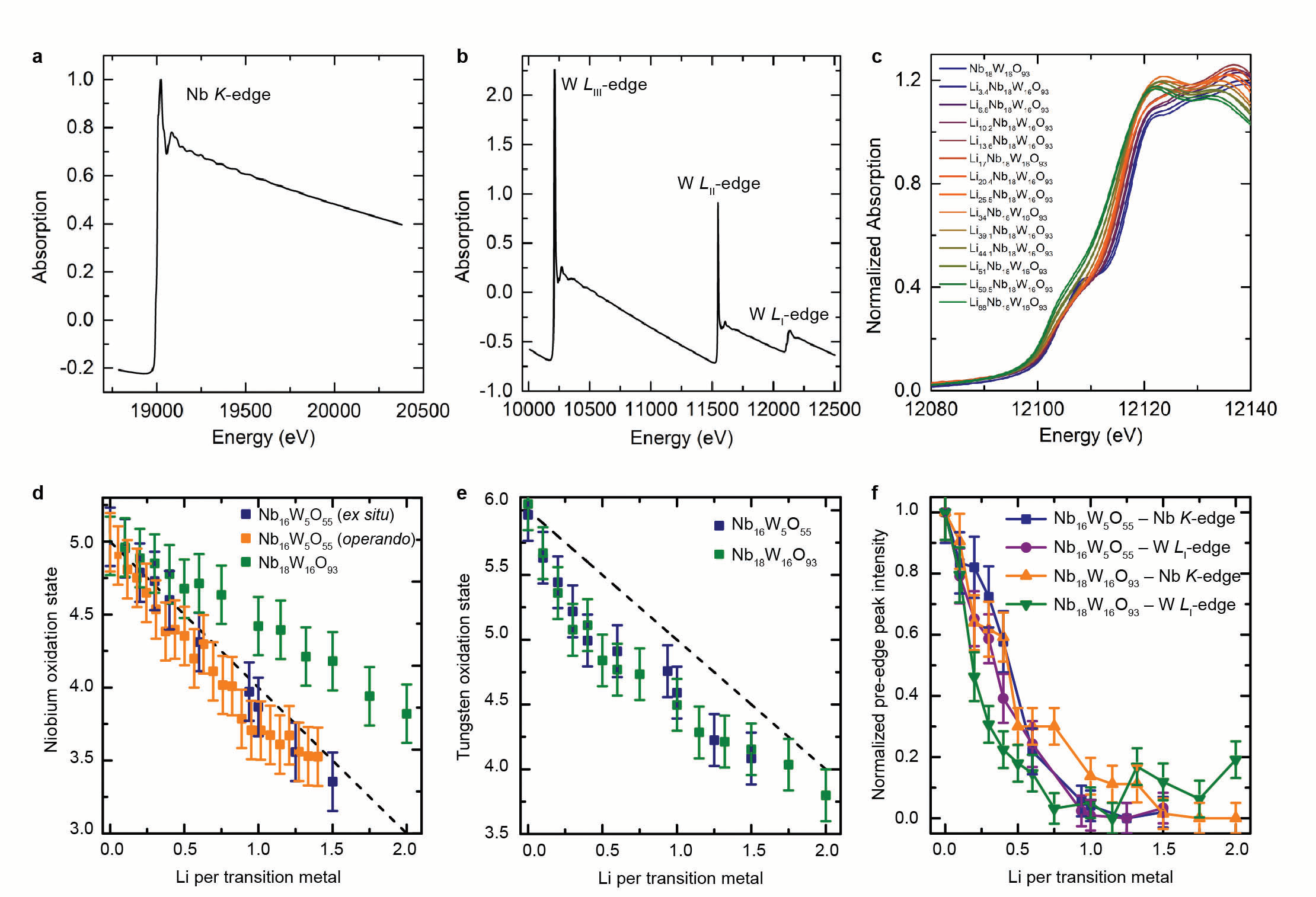
Tracking the absorption of X-rays at different states of charge led to the discovery that niobium and tungsten ions store charge simultaneously and both elements undergo multielectron redox and thus contribute to the high storage capacity of the new materials. Chemical insights from XAS were combined with lithium diffusion measurements from pulsed field gradient nuclear magnetic resonance spectroscopy and operando crystal structure analysis from X-ray diffraction during high-rate battery cycling. In conclusion, this multifaceted investigation provided a wholistic picture for advanced battery performance whereby these metal ions store excess charge, lithium is able to travel through the material with nearly liquid-like mobility, and the atomic structure undergoes minimal volume changes, thus minimal strain as it incorporates lithium. This research provides new electrode candidates for advanced battery technologies and proves that it is possible to achieve fast charging and high power operation with easily-produced large particles. The insights into the mechanism of operation also outline a strategy for future material discovery and design by identifying the key features of high-rate compounds.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.