Keep up to date with the latest research and developments from Diamond. Sign up for news on our scientific output, facility updates and plans for the future.
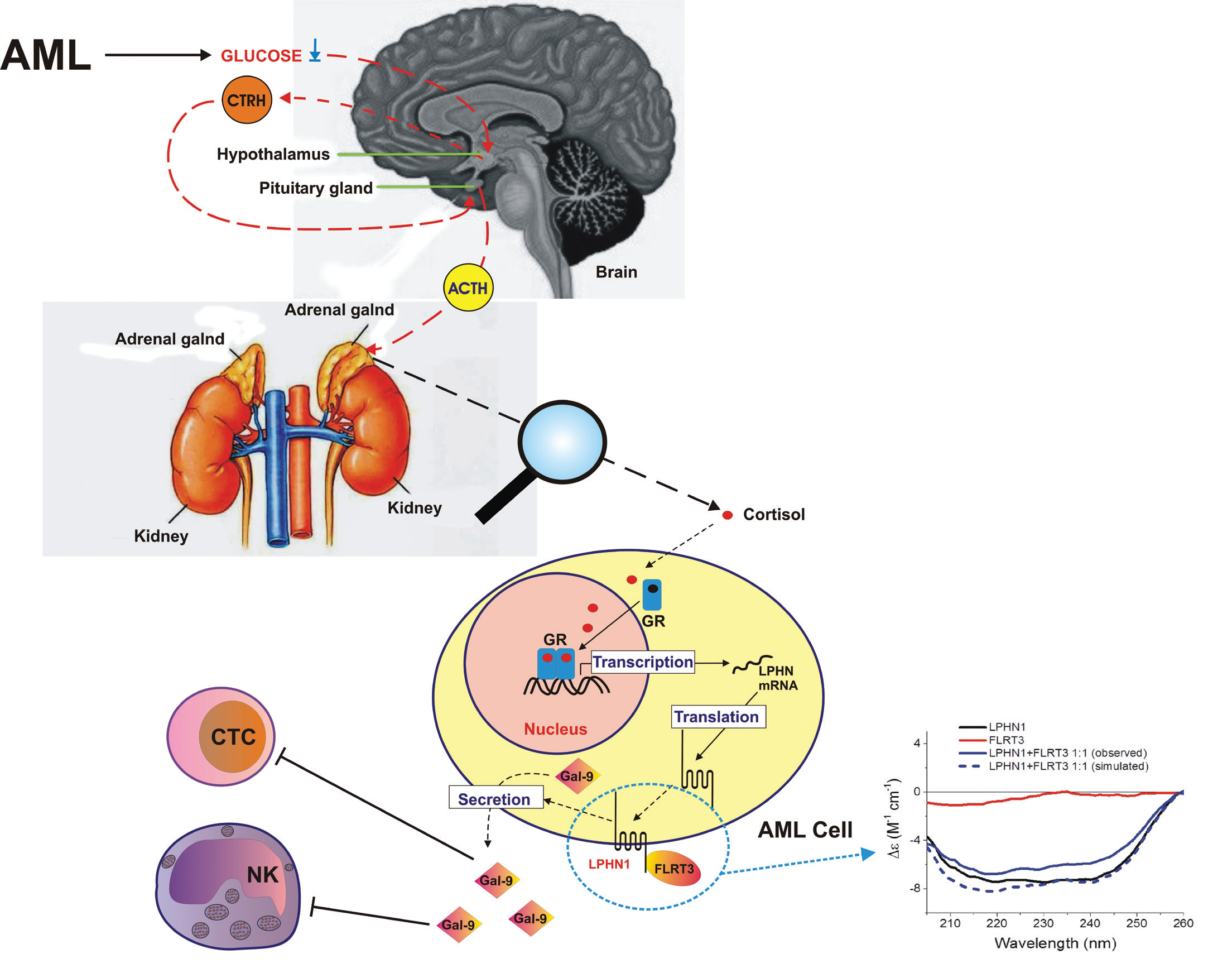
Acute myeloid leukaemia (AML) is a blood/bone marrow cancer which originates from human myeloid cell precursors, and rapidly develops into a systemic disease. AML cells can interact with cytotoxic immune cells of lymphoid lineage, such as natural killer (NK) cells and cytotoxic T cells (CTCs). AML cells can interact permanently with NK and CTC cells, thereby impairing the anti-cancer activity of these crucial immune cells. Unlike many other types of cancer cells, AML cells can promote anti-cancer activity not only during direct interaction with NK cells/CTCs, but also indirectly, by secreting immunosuppressive proteins like galectin-9, and its receptor and possible trafficker Tim-3 (T cell immunoglobulin and mucin domain containing protein 3). This pathway finally leads to impairing anti-cancer activity of NK cells, killing of CTCs, and even downregulation of their biochemical activation1. Our recent studies have shown that AML cells express a cell surface protein called latrophilin 1 (LPHN1), which is normally found in neurons, and also in haematopoietic stem cells (HSCs)1, 2. LPHN1 expression is repressed during HSC maturation, unless they undergo malignant transformation and become AML cells.
We found that cortisol, a small molecular weight steroid hormone (Fig. 1) produced by the adrenal cortex, can upregulate expression of LPHN1 in AML cells3, producing a significant increase in LPHN1 protein levels. Interestingly, cortisol levels are significantly increased in the blood plasma of AML patients compared to healthy donors. Respectively, galectin-9 levels in the blood plasma of AML patients was dramatically high compared to healthy individuals.
References:
Dr Vadim Sumbayev, University of Kent, [email protected] and Dr Rohanah Hussain, Diamond Light Source, [email protected]
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.