Keep up to date with the latest research and developments from Diamond. Sign up for news on our scientific output, facility updates and plans for the future.
Related publication: Zeng M., Meldrum F. C., Kim Y.-Y., Laundy D., Frontera C., Anduix-Canto C., Kapur N. & Christenson H. K. Confinement generates single-crystal aragonite rods at room temperature. Proc. Natl. Acad. Sci. 115, 7670–7675 (2018). DOI: 10.1073/ pnas.1718926115
Aragonite is a common crystalline form (polymorph) of calcium carbonate, an important biomineral found, for example, in seashells. Outside of the natural environment, aragonite usually only crystallises from solution at high temperatures, or in the presence of magnesium ions. However, although organisms can readily form aragonite, the way in which they do so remains unclear.
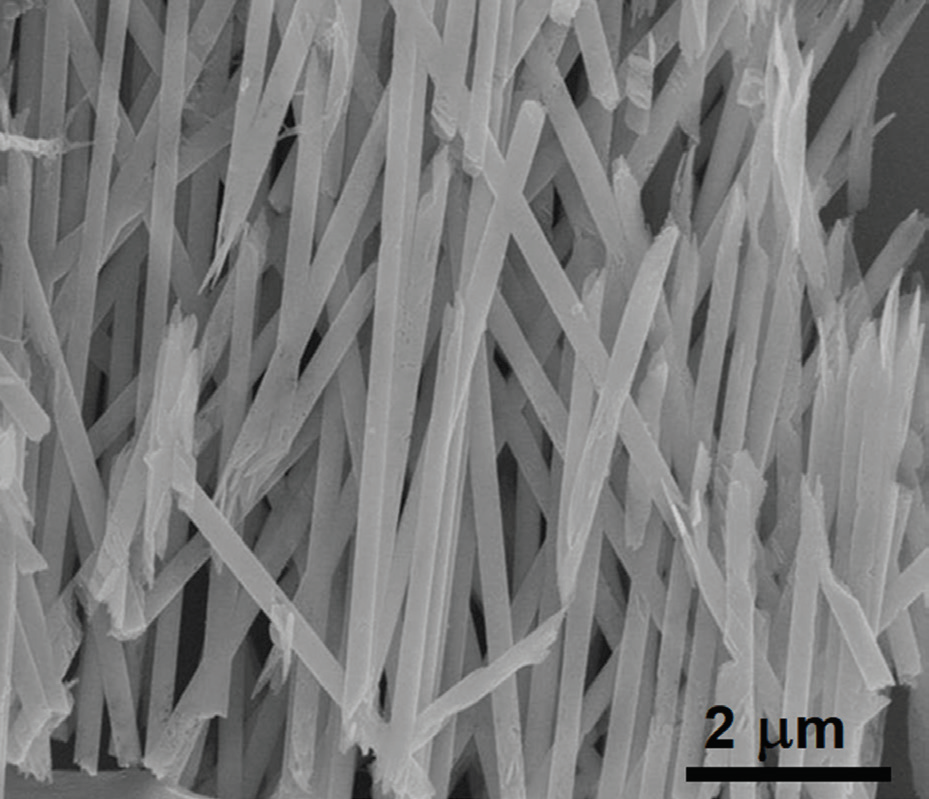
Organisms exhibit remarkable control over mineralisation processes, generating biominerals with complex morphologies, hierarchical structures, and outstanding mechanical properties1. However, while many of nature’s strategies for controlling biomineralisation are known, the mechanisms by which organisms control crystal structure are poorly understood. While it was long believed that the proteins entrapped within biominerals would hold the key, precipitation of calcium carbonate in the presence of proteins extracted from calcite or aragonite biominerals (where these are “polymorphs” of calcium carbonate, having the same composition but different crystal structure) failed to deliver polymorph control. The work described here explores an alternative explanation. It is well known that biominerals invariably form within small volumes, and that these can strongly influence crystallisation processes2,3. A simple system – crystallisation within the cylindrical pores of commercial filtration membranes – was therefore used to systematically investigate how confinement influences calcium carbonate polymorph.
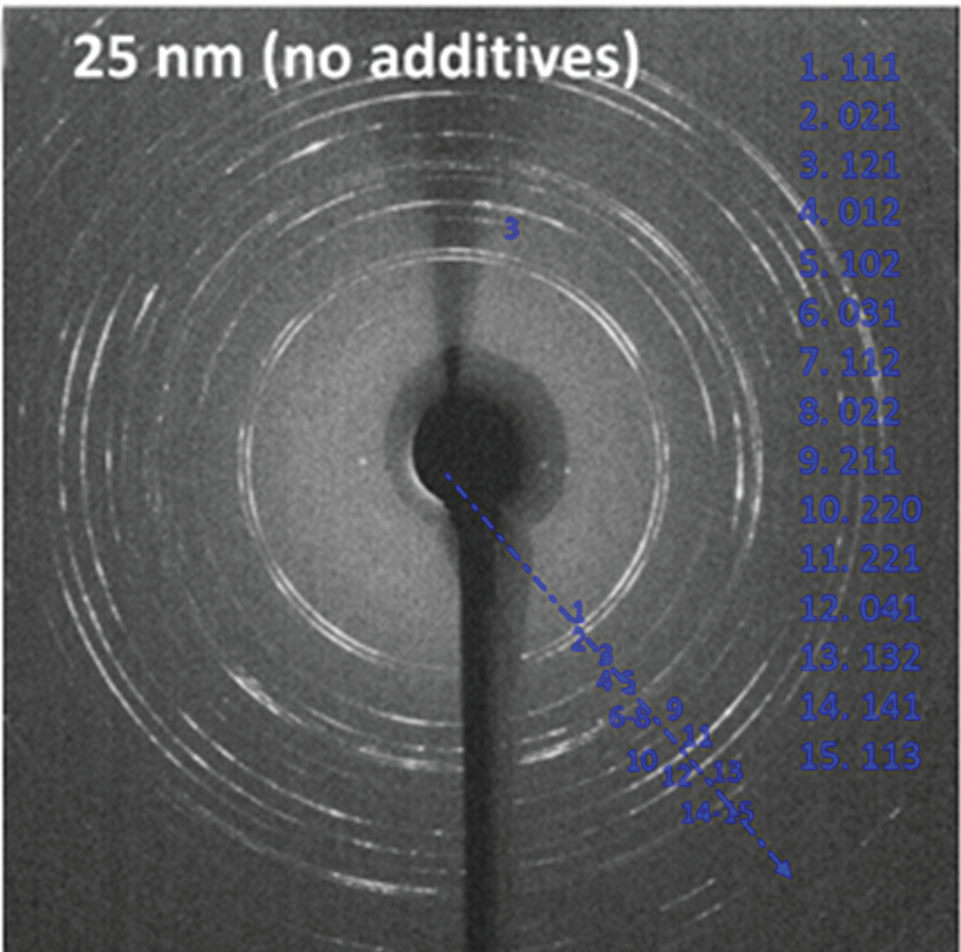
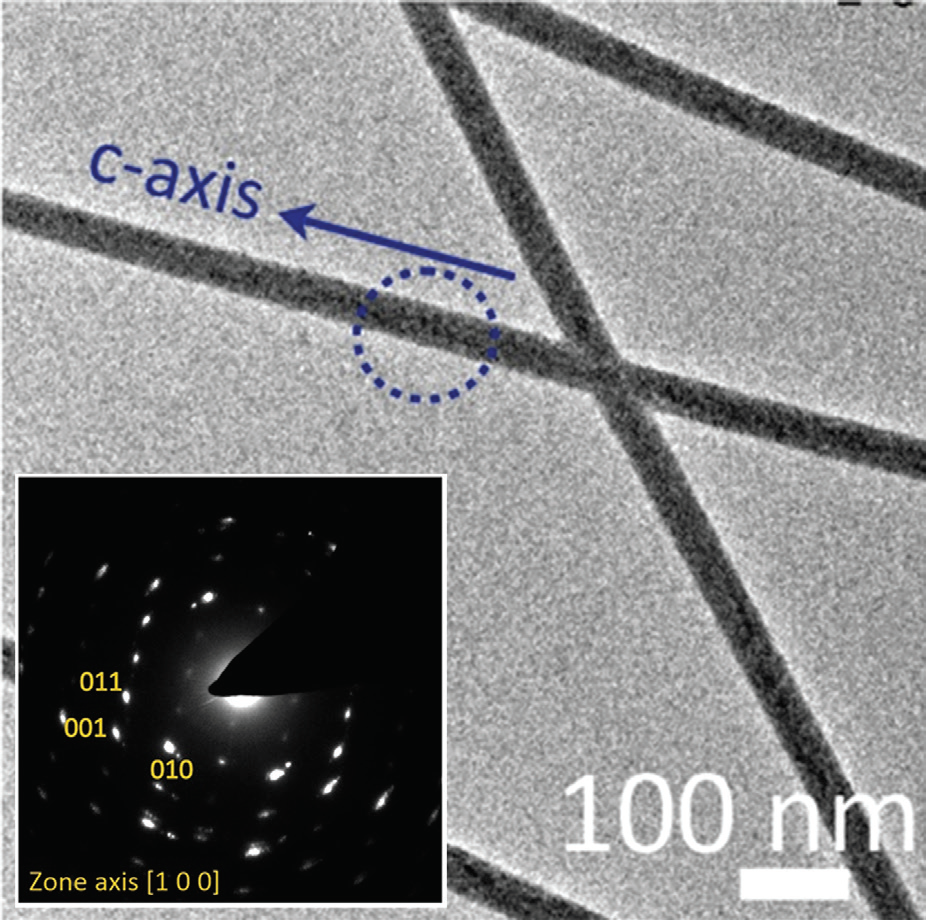
The possibility that the small pores affect the transport of ions to growing crystals – which may influence polymorph5 – was explored by modelling the diffusion of ions through the membrane pores. This showed that the calcium and carbonate solutions were fully mixed in under 0.1 sec, where mixing is rapid due to the short length of the pores. This cannot then be the origin of the polymorph change. The membrane surface may also have a significant influence on crystal formation. This was explored by analysing our data to explore the relationship between the aragonite fraction and the pore diameter. A graph of the aragonite fraction versus the inverse of the pore diameter revealed a roughly linear relationship between these quantities. This is expected if the number of aragonite nucleation sites is proportional to the surface area. Thus, as the surface increases in importance relative to the bulk with the degree of confinement, the proportion of aragonite increases. The distribution of ions adjacent to a charged membrane surface can also differ significantly in a membrane pore as compared to a planar surface, which may contribute to the effects seen.
References:
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.