 | ||
Surface reactions studied with X-ray standing waves |
Beamlines I09 Scientific Highlight
In recent years, the X-ray Standing Wave technique (XSW)1 has been employed to study the vertical bonding distance (dH) of molecules adsorbed on various single crystal substrates2. This high-precision technique (Δd ≈ 0.02 Å) exploits the element-specific photoemission signals to measure the adsorption heights of the different elements within the molecule. It uses the superposition of an incoming X-ray beam with its Bragg reflection to create an interference field with a periodicity of the substrate lattice planes (Fig. 1). The intensity profile of the electric field (maxima and minima) and the corresponding absorption of X-rays change in a characteristic way when scanning the photon energy (E) around the Bragg condition. By measuring the reflectivity of the sample and the variation of the photoelectron yield around the Bragg energy, the coherent position (PH), which is linked to the adsorption height of the adsorbates (dH), and the coherent fraction (fH), which is linked to the dispersion of these values, can be determined. The XSW technique has been successfully implemented to correlate the changes of electronic properties upon adsorption with differences in the geometry of the molecules3, becoming a valuable tool to study the metal-organic interface. The same rationale can be applied to effects induced by surface reactions. One particular case is the thermally induced self-metalation reaction that occurs when a molecule, after annealing at a certain temperature, incorporates a metal atom directly from the substrate upon which it is deposited. Here, the self-metalation reaction of 2H-tetraphenylporphyrin (2HTPP, see Fig. 2) on Cu(111)4 to copper(II)- tetraphenylporphyrin (CuTPP, see Fig. 2) was studied with X-ray Photoelectron Spectroscopy (XPS) and XSW on the Surface and Interface Structural Analysis beamline (I09), focusing on how the allocation of a copper (Cu) atom into the structure affects the adsorption geometry.
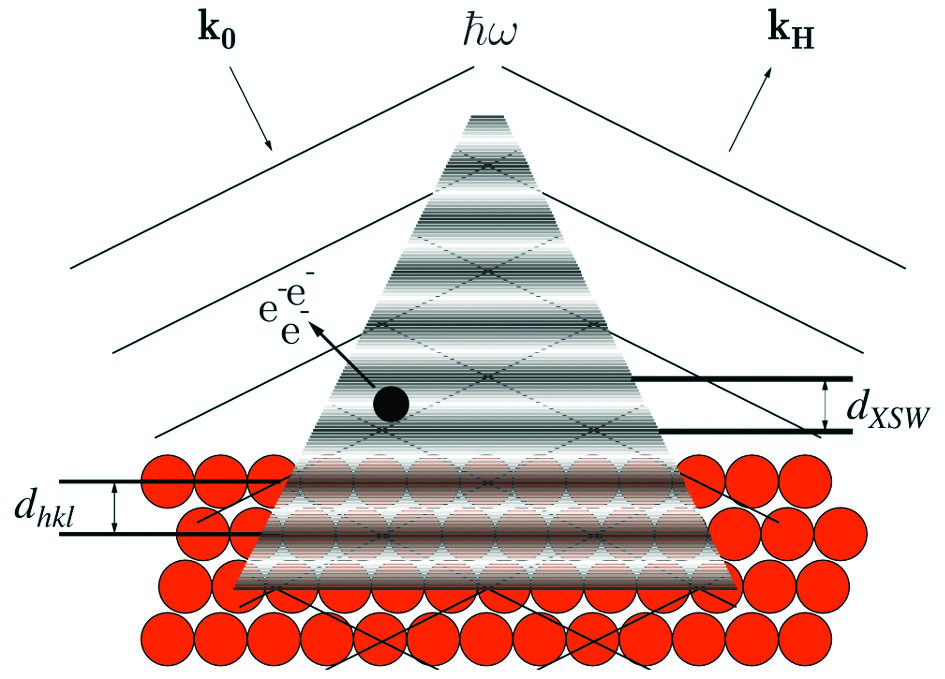
Figure 1: The superposition of the incoming (k0) and Bragg-reflected (kH) X-rays generates a standing wave field with maxima and minima that follow the periodicity of the crystal (spacing between layers, dhkl is the same as the spacing between nodes, dXSW). By scanning around the Bragg energy, the nodes and antinodes move and the photoelectron emission of an adsorbed atom (black dot) changes accordingly. The adsorption height of the atom to the substrate can be inferred by monitoring how the photoelectron emission changes.
XPS measurements were carried out at room temperature (RT) and at 146 K (LT). In both cases, the N1s core-level signal of 2HTPP shows two clearly distinguishable peaks4 (Fig. 3) arising from the two inequivalent nitrogen atoms (-N=, iminic and -NH-, aminic). Upon the temperature-induced metalation and the corresponding formation of (CuTPP), where all nitrogen atoms are coordinated to a Cu atom coming from the substrate, the XPS signal features a single peak (Fig. 3), because all nitrogens are now chemically equivalent.
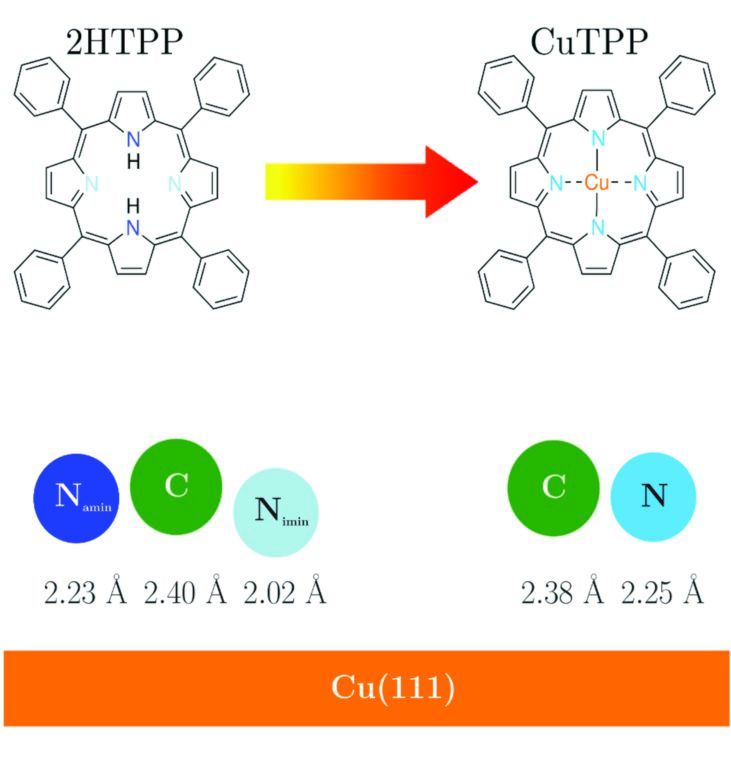
Figure 2: Schematics of the molecules studied and the corresponding adsorption heights as determined by XSW measurements. 2HTPP has two chemically inequivalent nitrogen atoms interacting with the Cu(111) substrate. The iminic nitrogen (-N=, light blue) adsorbs at a lower height than the aminic one (-NH-, dark blue), showing a stronger interaction with the substrate. Upon metalation at 500 K and the consequent formation of CuTPP, the differentiated behaviour disappears since all nitrogens are now equally coordinated to a Cu atom coming from the substrate. The carbon backbone adsorbs at almost the same height before and after the metalation.
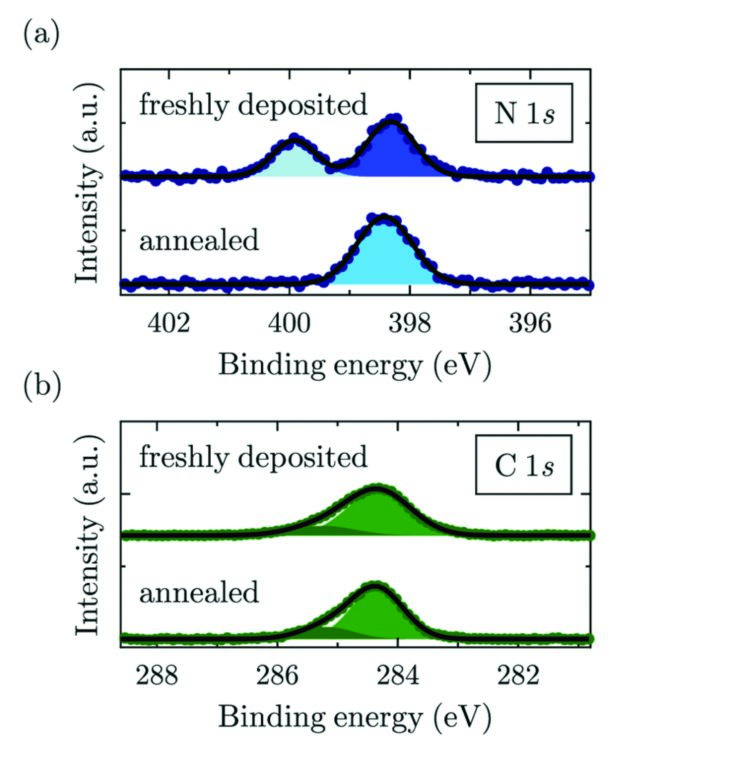
Figure 3: XPS measurements of (a) N1s (b) C1s core levels at RT before and after annealing. (a) shows how the metalation of the molecule upon annealing eliminates the inequivalent nitrogen atoms, the aminic (dark blue) and iminic (light blue) by equally coordinating them to the attached Cu atom; (b) shows no major changes in the C1s signal upon metalation. Two gaussian functions are used to fit the peak, one for the carbon atoms bound to other carbons or hydrogen and the other one for carbon bound to nitrogen. The two signals could not be resolved separately in the XSW data analysis.
XSW measurements using the C1s and N1s signals were carried out at RT (EBragg = 2.973 keV) and LT (EBragg = 2.980 keV), both for a freshly prepared and annealed sample. The annealing was performed at 500 K, i.e. at a relatively high temperature that yields a rather flat molecule due to the phenyl groups adsorbing mostly parallel to the surface5. Figure 2 summarises schematically the vertical bonding distances (dH) of the elements to the Cu substrate at RT. Here, only RT results are shown, since the trend at LT is the same. 2HTPP presents a distorted central ring having the iminic nitrogens closer to the surface (2.02 Å), which interact strongly with the substrate, in comparison to the aminic nitrogens (2.23 Å). After metalation, the equally-coordinated nitrogen adsorbs at 2.25 Å. It is interesting to note that in both cases the average carbon distance remains almost unaffected (from 2.40 Å to 2.38 Å). This shows that the incorporation of the Cu atom and the consequent elimination of the strong iminic nitgrogen-substrate interaction do not affect the overall adsorption height of the carbon skeleton significantly. A further complication is the possible rotation and bending of the four phenyl groups attached to the porphyrin ring. We can infer that upon annealing at 500 K, they are nearly parallel to the surface5. In addition, one can see that both for 2HTPP and CuTPP the average distance of the carbon atoms is slightly higher in comparison to those of nitrogen (~0.15 Å). It appears reasonable to attribute the higher adsorption distance of carbon to the phenyl groups being bent upwards. Using simple geometrical calculations and assuming that carbon atoms of the central ring adsorb at the average distance of nitrogen atoms, a bending of 10° is calculated for the phenyl groups of 2HTPP and 5° for the ones in CuTPP.
Finally, analysing the coherent fraction (fH) provides further information about the adsorption behaviour. fH, which ranges from 0 to 1 (0 standing for core-level signals coming from random distances to the substrate and 1 standing for core-level signals coming all of them from the same vertical distance), can be used to infer how the atoms are distributed around the average adsorption distance of the corresponding core-level within the molecule. For 2HTPP, C1s yields fH = 0.52 and increases to 0.71 for CuTPP. This increase shows a relaxation of the whole molecule, i.e. the carbons of the phenyl groups are adsorbing closer to those from the central ring, which is in line with the reduction of the bending angle. For the iminic nitrogen fH is 0.67 and for the aminic 0.72. Upon metalation fH increases up to 0.9 for the equallycoordinated nitrogen, which agrees with the picture of a relaxed central ring.
In summary, the surface reaction of self-metalation of 2HTPP on Cu(111) was successfully studied with XSW. The results show that the incorporation of the Cu atom and the consequent formation of CuTPP at 500 K do not show dramatic effects on the overall adsorption distance of the molecules. However, relaxation of the central ring and the flattening of the whole molecule are observed, since the phenyl groups are less strongly bent upwards and the suppression of the strong nitrogen-substrate interaction flattens the porphyrin ring.
Source publication:
Buerker, C., Franco-Canellas, A., Broch, K., Lee, T. L., Gerlach, A. & Schreiber, F. Self-Metalation of 2H-Tetraphenylporphyrin on Cu(111) Studied with XSW: Influence of the Central Metal Atom on the Adsorption Distance. Journal of Physical Chemistry C 118, 13659-13666, doi:10.1021/jp503046w (2014).
References:
1. Zegenhagen, J. & Kazimirov, A. X-ray standing waves in a nutshell. In The X-Ray Standing Wave Technique. Zegenhagen, J. & Kazimirov, A. (eds) pp. 3-35. World Scientific: London (2013).
2. Gerlach, A. et al. X-ray standing waves and surfaces x-ray scattering studies of molecule-metal interfaces. In The molecule-metal interface. Koch, N. et al. (eds) Ch.6. Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany (2013).
3. Heimel, G. et al. Charged and metallic molecular monolayers through surface-induced aromatic stabilization. Nature Chemistry 5, 187-194, doi:10.1038/nchem.1572 (2013).
4. Diller, K. et al. Self-metalation of 2H-tetraphenylporphyrin on Cu(111): An x-ray spectroscopy study. Journal of Chemical Physics 136, doi:10.1063/1.3674165 (2012).
5. Xiao, J. et al. Temperature-Dependent Chemical and Structural Transformations from 2H-tetraphenylporphyrin to Copper(II)- Tetraphenylporphyrin on Cu(111). Journal of Physical Chemistry C 116, 12275-12282, doi:10.1021/jp301757h (2012).
Funding acknowledgements:
This work was carried out with the support of Diamond Light Source. This work was financially supported by the DFG (SCHR700/14-1).
Corresponding author:
Dr Alexander Gerlach, Universität Tübingen, [email protected]


 A brighter light for science
A brighter light for science
