 | ||
A perfect fit: Understanding how porous organic cages separate rare gases and chiral molecules |
Beamlines I19/I11 Scientific Highlight
Two techniques were used at Diamond Light Source to analyse the organic cage molecule CC3 as gas was adsorbed into the material. The High Resolution Powder Diffraction beamline (I11) enabled measurements to be made in the capillary cell whilst the gases were adsorbed, and the Small Molecule Single Crystal Diffraction beamline (I19) allowed detection of the binding molecule due to its high resolution in studying small crystals. Comparison of the binding of xenon (Xe) and krypton (Kr) in CC3 showed that the crystal structure does not rearrange significantly but that more Xe is adsorbed than Kr. This agrees with simulations performed, confirming predictions that CC3 preferentially binds Xe over Kr. The success of this methodology highlights the potential for larger cage molecules to be used for separations of biomolecules.
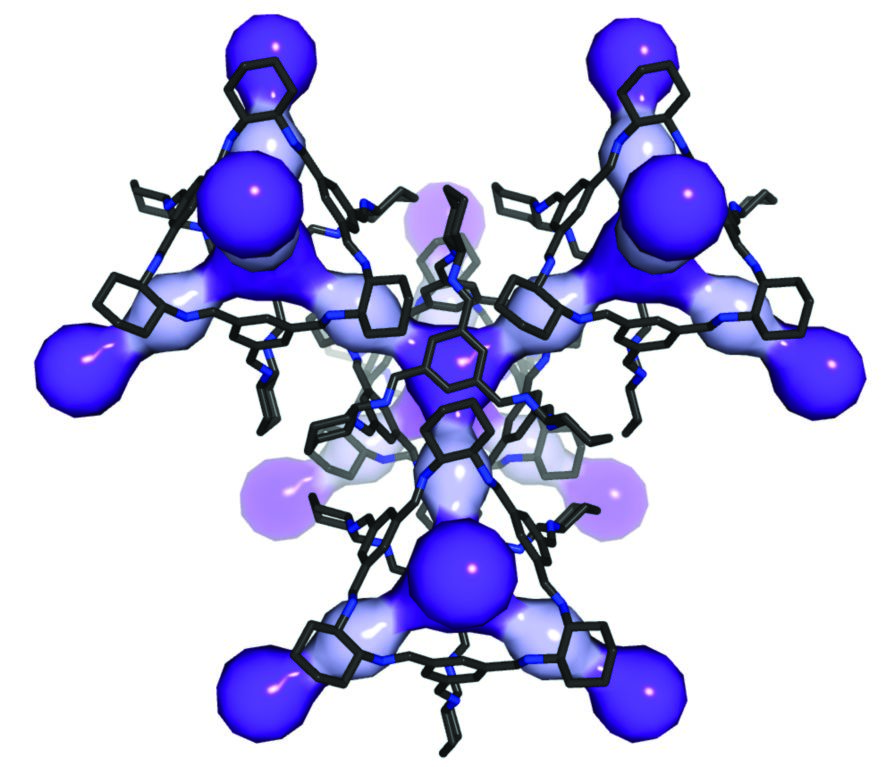
Figure 1: Crystal structure of CC3 showing the 3-D connected diamondoid pore network, coloured to highlight two types of cavity: intrinsic cage pores (dark purple) and a smaller window cavity formed between two adjacent cage windows (light purple).
CC3 is chiral organic cage molecule that is prepared in pure -R or -S form1. The cage has an internal cavity that is surrounded by four windows with a tetrahedral arrangement. In the solid state, CC3-R (or CC3-S) packs such that the internal cage cavities are connected via the four cage windows, which form the narrowest point in the resulting diamondoid pore network (d = 3.6 Å, Fig. 1). Previous research has shown that the geometry and dimensions of this diamondoid pore network allows the selective adsorption of small aromatic structural isomers on the basis of size and shape2. Chiral separations pose an alternative challenge, and the inherent chirality of the diamondoid pore network in crystalline CC3-R suggested the possibility for such separations.
This cage can also be used to separate gas molecules by size. With the exception of argon, rare gases occur naturally in the air at very low concentrations. They are commercially valuable due to their medical and technological applications, but their unreactive nature, low concentration, and similarity in size to the other constituents of air makes them difficult to extract. Some rare gas isotopes can cause damage to health, such as naturally occurring radon gas (222Rn) which is a leading cause of lung cancer. Harmful radioisotopes of Xe and Kr generated by nuclear fission are also released into the atmosphere in low concentrations during reprocessing of spent nuclear fuel or as a result of nuclear accidents.
The spherical atomic nature of the rare gases also means that their shape selective separation is not possible. The intrinsic pore of CC3-R is very similar in size (d = 4.4 Å) to Xe and radon (4.10 and 4.17 Å, respectively), but the static window diameter is slightly smaller than Kr (3.69 Å), and as such, CC3-R crystals should be non-porous to these gases. By contrast, molecular dynamics simulations show the flexible cage window transiently ‘opens’ to allow diffusion of Xe and Kr3. Experimental and simulated isotherms confirmed that CC3-R is highly porous to both Kr and Xe. Moreover, simulated isotherms and experimental measurements show that CC3-R also exhibits high radon uptake. A number of metal-organic frameworks (MOFs) have shown promising Xe/Kr separation4, but the search continues for materials with high uptake and selectivity at low concentration and non-cryogenic temperatures, and CC3-R is currently one of the lead contender materials for this application.
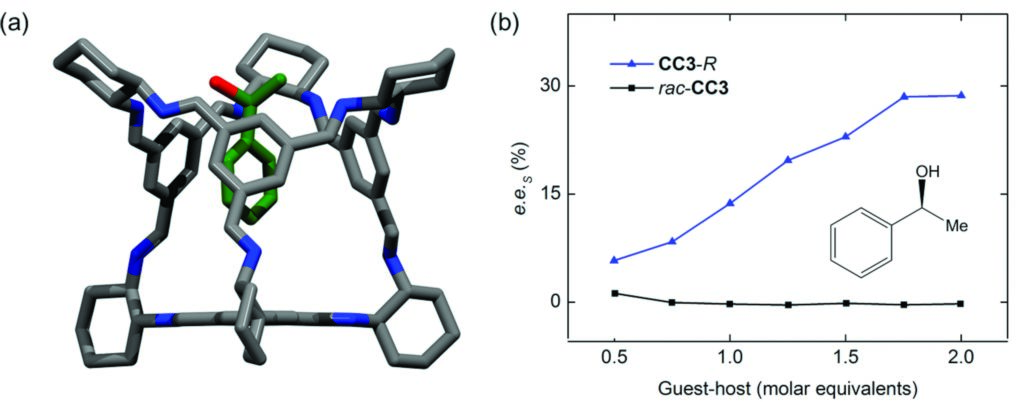
Figure 2: (a) Host-guest crystal structure showing (S)-1-phenylethanol residing in the intrinsic cavity of CC3-R; (b) measured enantiomeric excess of the S enantiomer of 1-phenylethanol (e.e.s) adsorbed by CC3-R over a range of guest-host ratios; adsorption of 1-phenylethanol by racemic CC3 crystals was not enantioselective
To investigate how Xe and Kr interact with the CC3-R crystal, in situ crystallography data was collected on beamlines I19 and I11 at Diamond. In situ Powder X-ray Diffraction (PXRD) data collected at beamline I11 using the low pressure capillary gas cell5 up to 10.1 bar Xe at 295 K showed the guest was reversibly adsorbed with little rearrangement of the CC3-R molecules. Under these conditions, CC3-R accommodates up to 2.8 Xe atoms per cage (Fig. 3a) (1.87 mol kg-1, 25 wt. %), with a small preference for the internal cage pore (Fig. 3b) over the window cavity (Fig. 3c). The lower guest occupancies observed in Kr-loaded CC3-R are consistent with its lower affinity than for Xe.
Breakthrough measurements were carried out at the Pacific Northwest National Laboratory in the USA to assess the potential of CC3-R in real separations of rare gases at low concentrations in air. A simulated air mixture was passed through an adsorption column packed with crystalline CC3-R. The main components of air broke through the column almost immediately, as did Kr (40 ppm). In contrast, Xe was retained on the column for over 15 minutes, even at an increased flow rate of 40 cm3 STP min-1. Under these conditions, CC3-R outperforms a leading MOF, Ni-DOBDC4, with twice the Xe uptake (11 mmol kg-1) and almost thre- times higher Xe/Kr selectivity (20.4 vs. 7.3).
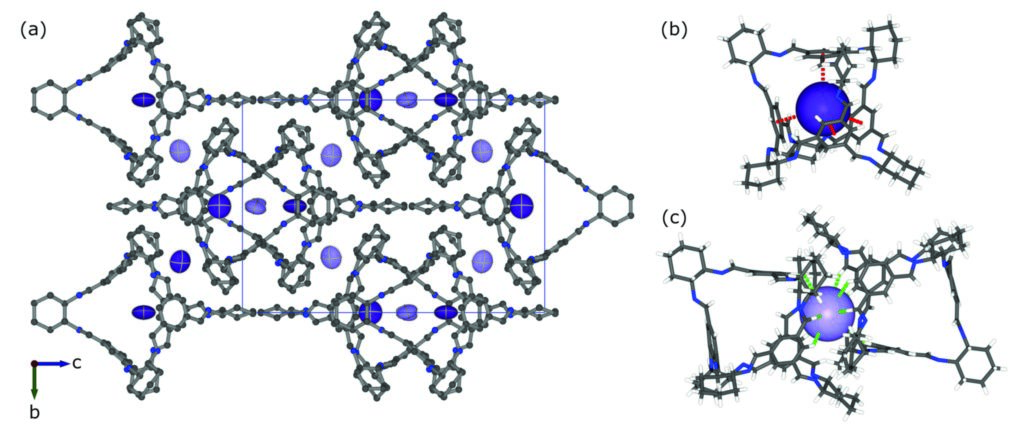 Figure 3: (a) Crystal structure of CC3·2.8Xe at 10.1 bar of xenon and 295 K, viewed parallel to [100]; (b) full site occupancy suggests the xenon guest exhibits a small preference for the larger intrinsic cage pore, where the shortest contact distances are between Xe and the phenyl ring (mean rXe-Cg = 4.2 Å); (c) Weak Xe – H contacts (mean rXe-H = 3.75 Å) are present in the less occupied window cavity.
Figure 3: (a) Crystal structure of CC3·2.8Xe at 10.1 bar of xenon and 295 K, viewed parallel to [100]; (b) full site occupancy suggests the xenon guest exhibits a small preference for the larger intrinsic cage pore, where the shortest contact distances are between Xe and the phenyl ring (mean rXe-Cg = 4.2 Å); (c) Weak Xe – H contacts (mean rXe-H = 3.75 Å) are present in the less occupied window cavity.
Source publication:
Chen, L., Reiss, P. S., Chong, S. Y., Holden, D., Jelfs, K. E., Hasell, T., Little, M. A., Kewley, A., Briggs, M. E., Stephenson, A., Thomas, K. M., Armstrong, J. A., Bell, J., Busto, J., Noel, R., Liu, J., Strachan, D. M., Thallapally, P. K. & Cooper, A. I. Separation of rare gases and chiral molecules by selective binding in porous organic cages. Nature Materials 13, 954-960, doi:10.1038/nmat4035 (2014).
References:
1. Tozawa, T. et al. Porous organic cages. Nature Materials 8, 973-978, doi:10.1038/nmat2545 (2009).
2. Mitra, T. et al. Molecular shape sorting using molecular organic cages. Nature Chemistry 5, 276-281, doi:10.1038/nchem.1550 (2013).
3. Banerjee, D. et al. Potential of metal-organic frameworks for separation of xenon and krypton. Accounts of chemical research 48, 211-219, doi:10.1021/ar5003126 (2015).
4. Holden, D. et al. Gas Diffusion in a Porous Organic Cage: Analysis of Dynamic Pore Connectivity Using Molecular Dynamics Simulations. Journal of Physical Chemistry C 118, 12734-12743, doi:10.1021/jp500293s (2014).
5. Parker, J. E., Potter, J., Thompson, S. P., Lennie, A. R. & Tang, C. C. in Thermec 2011, Pts 1-4 Vol. 706-709 Materials Science Forum Chandra, T., Ionescu, M. & Mantovani, D. (eds) pp 1707-1712 (2012).
Funding acknowledgements:
The research leading to these results has received funding from the ERC (Grant Agreement no. 321156, RobOT), EPSRC (EP/H000925/1; EP/K018396/1) and Region PACA (Provence-Alpes-Cote-d’Azur). K.E.J. is a Royal Society University Research Fellow. We thank Diamond Light Source for access to beamlines I19 (MT8728) and I11 (EE7040). The work at PNNL was supported by US Department of Energy (DOE), Office of Nuclear Energy.
Corresponding author:
Professor Andrew I. Cooper, University of Liverpool, [email protected]


 A brighter light for science
A brighter light for science
