Find out more about our ambitious upgrade project, delivering more brightness, more coherence, and greater speed of analysis to UK science. More about Diamond-II
![]()
Find out more about Diamond's response to virus research.
![]()
Scientists from Imperial College London have used data collected at Diamond Light Source to advance the understanding of how HIV and other retroviruses infect human or animal cells. The research, which is funded by the Medical Research Council, was published in Nature this week.
Using Diamond’s finely tuned pinpoint X-ray beams the researchers were able to determine 3D structures of the key molecular machine used by viruses, such as HIV, to insert copies of their genetic material into host DNA.
This fundamental knowledge will not only facilitate design of better drugs for fighting AIDS, but may also have an impact on pioneering treatments such as gene therapy; an experimental technique using tamed versions of viruses to treat genetic disorders, such as “bubble boy” disease – a condition where a defective gene results in its sufferers having little or no immune system making them extremely vulnerable to infectious diseases and in some cases having to permanently live inside a sterile environment.
 View animation of the integration mechanism
View animation of the integration mechanism
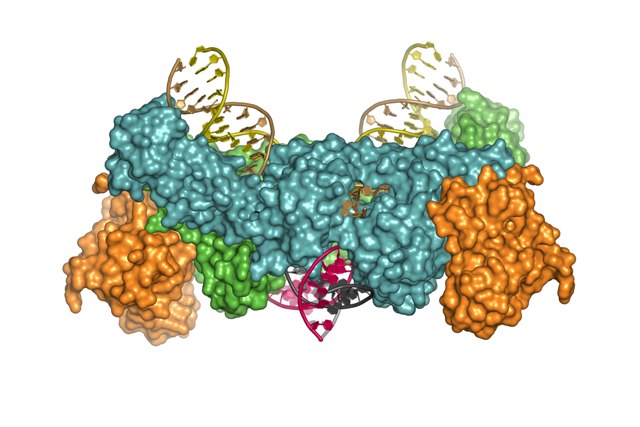
“It has truly been a breathtaking ride. Only 18 months ago we had a rather sketchy understanding of retroviral integration. Now we have obtained snapshots depicting the whole process in atomic detail. The new 3D structures capture the retroviral integration machine in action.”Dr Peter Cherepanov, Imperial College London
The structural data collection was carried out on the I02 macromolecular crystallography experimental station at the Diamond synchrotron in Oxfordshire.
“This kind of fundamental research is vital if we are to advance our understanding of the viruses and diseases that affect millions of people around the world. Knowing the 3D structure of the mechanisms involved is like being able to see inside the engine of your car. If you can actually see what is happening, you get an idea of how you can fix it. At Diamond we produce the extremely intense X-ray beams required for looking at the molecular interactions involved in a variety of biological processes.”Professor Thomas Sorensen, Diamond Light Source
Advances in structural biology have accelerated greatly as a result of access to the synchrotron facilities that have been developed around the world in the past 25 years. Biologists have been swift to recognise the huge potential that lies behind understanding the multitude of processes that take place within living organisms at a molecular level. Researchers in the UK are at the forefront of this work and Diamond Light Source plays its part in providing cutting edge facilities for protein structure determination. Diamond currently has five experimental stations dedicated to structural biology as well as an on-site membrane protein laboratory. Previous breakthroughs using structural data from Diamond include gaining a better understanding of hypertension in the pre-natal condition pre-eclampsia, learning how a key tuberculosis drug is activated, understanding how bird flu can affect humans, and solving the 3D atomic models of a single transporter protein responsible for the movement of essential chemicals into cells of the body.
The mechanism of retroviral integration through X-ray structures of its key intermediates, Goedele N. Maertens, Stephen Hare & Peter Cherepanov, Nature November 2010.
DOI 10.1038/nature09517
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.