Keep up to date with the latest research and developments from Diamond. Sign up for news on our scientific output, facility updates and plans for the future.
Related publication: Sanchez-Cano C., Romero-Canelón I., Geraki K. & Sadler P. J. Microfocus x-ray fluorescence mapping of tumour penetration by an organo‑osmium anticancer complex. J. Inorg. Biochem. 185, 26–29 (2018). DOI: 10.1016/j.jinorgbio.2018.04.014
Some of the most effective cancer treatments involve platinum-based drugs, but resistance to platinum compounds is increasing and there is an urgent need to find alternatives. A team from the University of Warwick has discovered a new osmium-based (Os-based) anti-cancer agent, FY26, which exhibits high potency against a range of cancer cell lines and is capable of overcoming platinum resistance. Before it can be tested in clinical trials, the team needs to understand fully how it works.
Tumours are complex systems that show a low level of irrigation by blood vessels. Poor penetration can limit the ability of a drug to reach the interior of a tumour, which is of vital importance for the success of most anti-cancer agents. The researchers wanted to know how FY26 would behave in tumours, and in particular if the drug would be able to penetrate easily into their core. To investigate, they used the Microfocus Spectroscopy beamline (I18) which can resolve individual cells within a model tumour (a collection of closely packed cancer cells known as a spheroid) and has high sensitivity to detect the drug at low concentrations.
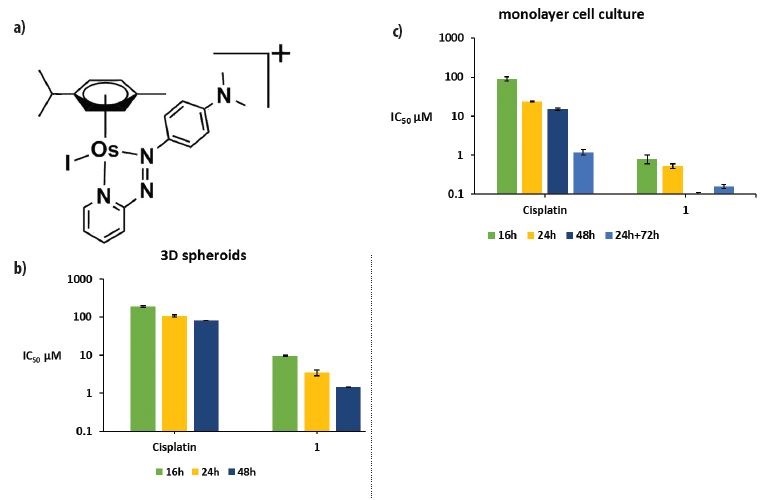
According to the World Health Organisation, cancer is the second leading cause of death globally (about 1 in 6 deaths), and was responsible for an estimated 9.6 million deaths in 2018. Furthermore, approximately 70% of deaths from cancer occur in low- and middle-income countries1. Currently, some of the most effective cancer treatments involve platinum-based drugs, which are used in almost half of all cancer patients who need chemotherapy. However, resistance to platinum compounds is increasing, and there is an urgent need to find alternative anticancer agents2.
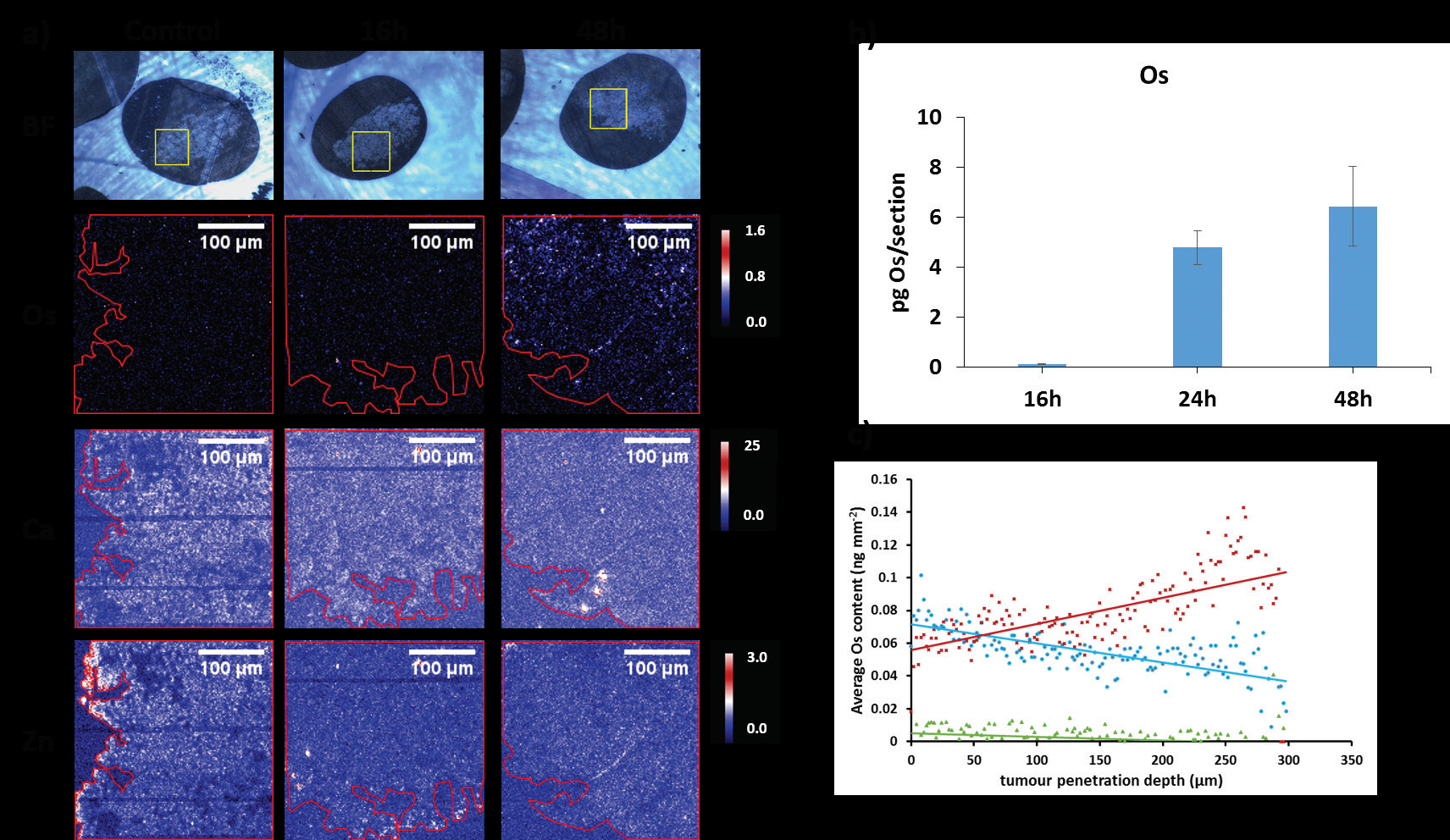
These maps confirm the presence of Os in samples treated with the drug. FY26 complex is capable of penetrating into the tumour core, and remains there for longer than expected. Furthermore, the measurements show that both tissue penetration and accumulation of Os is directly related with the time of spheroid exposure to the drug (Fig. 2). In contrast, cell monolayers showed maximum uptake after 24 h incubation, followed by a rapid decrease in the amount of Os inside cells during the next 48 h. These differences in the accumulation kinetics of FY26 between cell monolayers and 3D tumour models may result from more effective efflux from cell monolayers, or slow diffusion of the drug into tissue-like samples.
Treatment of spheroids with the Os drug also leads to extensive changes in Zinc (Zn) and Calcium (Ca) distribution, from being discretely localised in untreated samples to being more widely distributed in treated spheroids (Fig. 2a). This change in distribution is time-dependent. After 16h exposure to the drug, some Zn and Ca are still discretely localised in certain areas of the spheroids, whereas after 24 or 48 h incubation, they are more evenly distributed. The changes observed in the Zn and Ca maps of spheroids are in good agreement with those observed for monolayer cultured cells treated with the drug5. Overall, these alterations confirm the presence of nuclear damage with Ca release from the endoplasmic reticulum (ER), possibly indicating immunogenic cell death, which remains to be further elucidated.
Overall, these microfocus X-ray fluorescence experiments on beamline I18 provide new insights into the interaction of a promising organo-osmium anticancer drug candidate with solid tumours. XRF maps show that the Os drug penetrates efficiently into tumour spheroids, although more complicated models would be required to mimic the heterogeneous nature of solid tumours. Interestingly, such studies could also be made using the I18 microprobe, on real tumours extracted from animal models treated with this drug candidate. Nevertheless, the results highlight the potential of this organo-osmium complex as a candidate for further development as a clinical drug to treat platinum resistant tumours, a current clinical need.
References:
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.